| Pages:
1
2
3
4
5
6
..
48 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@MadHatter
Those welding carbons are a sort of a partially graphited porous carbon material , I think , IIRC they are perhaps a blend of something like a ceramic
cement , maybe an oxychloride cement with bone? charcoal which has been pressed into sticks and then high fired until it has a partially fused almost
ceramic composite graphite/charcoal composition not nearly as dense as say
solid graphite like used for motor brushes .
Have you ever tried the more dense and non-porous
solid graphite material to see if it holds up any better than the porous type material ?
Also I have wondered about a coaxial electrode arrangement where the carbon or graphite anode is
placed inside a length of stainless steel pipe , perhaps
held centered in the pipe with spacers or nylon screws
on a 120 degree pattern through the stainless pipe
around the periphery near each end .
It would seeem that such an electrode placed vertically
or tilted a bit , would perhaps act as its own liquid pump
pulling fresh electrolyte across itself from any rising escaping bubbles on its inside surfaces .....along the same lines as the entrainment flow
works for an aquarium filter .
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
If you want a self pumping electrode assembly try to imagine this: Co-axial electrodes supported by a helical spacer, which forms an archimedes screw
sort of thing. Now wrap a solenoid winding around the whole thing - could even use the electrolysis current to power the solenoid. What you get is a
radial current flowing in an axial magnetic field - resulting in a tangential force on the electrolyte. The helical spacer makes the resulting
swirling electrolyte "screw" its way throught the whole assembly - assuming an inlet at one endof the helix and an outlet at the other. Voila! self
pumping electrolysis!
It occurs to me that the solenoid could also be conveniently replaced with loudspeaker magnets, which have a lovely hole through the middle, and are
magnetised axially.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
How about just using a stainless steel tubing coil for the cathode , and then possibly you would get the field
as if it were windings , and you could also put cooling water through it .
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
To echo Rosco Bodine :
Bubbles alone will give you self-pumping. Even with a non-enclosed electrode there will be some upwards flow of fluid, put a tube around it and the
pumping action is enhanced. Consider how many under-gravel filters in aquariums work, the do a web search for "air lift" or airlift pumps. No
messing around with rotating parts, which can be annoyingly difficult to keeep functional in a corrosive environment.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
not_important, you mis-understand. There are no moving parts. Its a bit like this :MHD drive
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Twospoons
not_important, you mis-understand. There are no moving parts. Its a bit like this :MHD drive |
You are correct, I did - haven't really woken up. However I think that MHD is hardly going to give a compact, simple pump. I think that either the
intrinsic gas release, or blowing air or N2 through the tube, will work as well with a lot less fuss. The only place MHD seems to be used for pumping
is with liquid metals or on a micro scale.
It's be a fun project, but I'd not mess with it if I wanted to actually use the cell for production.
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
It actually works better than you'd expect. I tried a very crude setup with a ferrite magnet under a dish of saline, put two electrodes in, and ran a
couple of amps through. With no attempt at optimising, the saline swirled nicely around the electrodes.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
just a quick (possibly helpfull) tip.
for those using Gouging rods, after you peel off the copper plate/foil, soak the electrodes in dillute nitric acid (2%) for several hours before using
them, it gets rid of any remaining copper metal in the pores (and there is some!).
then wash them off in plain water and leave them somewhere warm and dry, I put mine on the radiator.
it`s just one LESS source of possible contamination 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by jpsmith123
For a cell body, I used a 1 L pyrex graduated cylinder, and for a lid, I used a slightly modified plastic lid from a jar of Skippy peanut butter,
which held the electrode assembly.
The disadvantage of the "Salchlor" anode is the length; at 12 inches long, it is hard to find suitable containers that can accomodate the whole
electrode. In retrospect, I think it would have been better to go with the "Autochlor" AC-20 or AC-25 chlorinator cell assembly.
Anyway, I filled the cell with saturated NaCl solution and ran about 15 amps through it, but it made more chlorine gas than I was expecting, and I was
afraid it would bother the neighbors, so I shut it down  . .
I decided to put the project temporarily on hold, as I don't really have a good place to work right now. Hopefully, in a few more weeks, that
situation will change, and I'll be back in business.
(In the meantime, apparently someone on the E&W forum has used a pool chlorinator anode similar to mine, to make a few lbs. of NaClO4, apparently
in a batch type process. I'm thinking maybe someone registered on both forums can ask that poster for the specifics of his cell arrangement and
process and post any useful information here). |
Hello,
Reading this post I was surprised to learn that someone had make Perchlorate using a pool chlorination anode. (MMO) . Then perhaps the anode was
platinum or PGM based.
Anyone here know?
Cheers,
Dann2
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hello Dann2,
Several people have reported using pool chlorinator anodes to make ClO4. These anodes have MMO coatings, usually RuO2 + TiO2, and sometimes RuO2 +
IrO2 + TiO2, which may be more robust. Apparently PdO2 + TiO2 works well also...in fact it may have better catalytic activity than the other PGM
oxides, but I think it is somewhat more expensive than RuO2 and thus not generally used in pool chlorinators, IIRC.
BTW, I think I remember reading in one of Beer's patents where he claims to have successfully used graphite as a substrate for his MMO coatings.
I recently built a new cell based on a 20 amp "autochlor" chlorinator, but I have not been using it because right now I live in a very small place in
a lousy area not at all conducive to experimentation.
Regards,
jpsmith123
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Some info.
Reasons for the DSA Passivation during Chlorate Electrolysis and the Means for Extending the Anode Service Life.pdf
Dann2
Attachment: Reasons for the DSA Passivation during Chlorate Electrolysis and the Means for Extending the Anode Service Life.pdf (56kB)
This file has been downloaded 1934 times
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Some info. on Sodium Chlorate making here:
Encyclopedia of Chemical Processing and Design.
John J. Mc Ketta
http://books.google.ie/books?id=_R00NqWST6MC&pg=PA233&am...
There is some info. on DSA anodes. It says that Barium will poison them.
Missing pages here:
http://ifile.it/6gy0ish
Have a good bedtime read!!!
Dann2
[Edited on 2-10-2008 by dann2]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
O Deary me,
You live and learn :-|
I have just realized that Google books switch around the 'missing pages' on there books, therefor the 'missing pages' that are at the ifile.it link
are no longer missing but a different set are  #~~@f%$~#~#.. *7*&+##
!!!! #~~@f%$~#~#.. *7*&+##
!!!!
Is there an easy way to download books from Google books (bits of books). You can go back a week later and download the missing (on day one) pages.
You could always use the PrtSc button but it is a bit of a pain, cheap though.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Obtained "The effect of pH on graphite wear in a chlorate cell process", thanks to folks in Wanted refs and translations in the refs. section. It
gives the lowdown on Graphite erosion in Chlorate cells operated at very high pH. Up at 9 - 10 where non-pH controlled cells operate (never came
accross this info. before).
It also states that Graphite from the anode is mainly lost as sludge (read: black mess) when cell is operated at high pH, as opposed to converting to
CO2 when cell is operated at around neutral (as per industry).
pH control looks attractive (greater CE, less erosion per amper hour and per KG Chlorate, less actual eroded C appears as sludge) especially since it
would appear that *control* as such is not really needed. Just adding HCl at a predetermined rate and checking pH twice per day (or once) will give a
tight enough pH range.
The figures from Industry for Graphite erosion in Chlorate cells have always been very impressive. A graph from the article is below.
Dann2
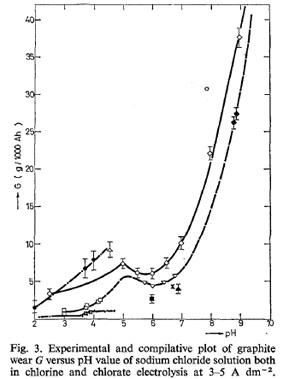
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ Dann2
That's an amazing graph, it explains so much!
If I remember correctly, my chlorate cells were operating in the 9 - 10 pH range 
Has anyone tried the "cheap" aquarium pH controllers like the WEIPRO PH2010 ?
http://www.deepblueaquarium.co.nz/index.htm
(Check in the controllers and monitors section - the direct link doesn't work for some reason)
I gather they can be obtained for less than US$100.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Xenoid,
Long time no see!
I would think that the pH probe in the aquarium controllers may not stand up to the (rather more) harsh environment in the Chlorate cell.
Look what it can do to most plastics etc, what would it do to your Guppy I ask???
Also in the article mentioned above, it gives some stuff for treating Carbon with (we usually use Linseed oil).
Industrial Graphite for Chlorate cells must be porous.
Quote:
__________________________________
It might also be added that the impregnation
method described elsewhere [3] has been
developed still further, and the improvements
obtained with diacyl peroxides, and, particularly,
with dilauroyl peroxide [3], were advanced with
the use of pinan hydroperoxide [43]. The latter
is stable to higher temperatures thereby providing
the benefits described elsewhere [3], that result
from keeping the anode potential close to the
value for plain graphite and from increasing
the life-times of electrodes in a chlorate cell
process.
___________________________________
http://www.arkema-inc.com/index.cfm?pag=69
At least it's not toxic!
pinan hydroperoxide
diacyl peroxides
dilauroyl peroxide
Don't suppose they are available in hardware store etc.
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ Dann2
Yeah, Hi! Lost interest, I've got so much chlorate and perchlorate I don't know what to do with it!
I assume the pH probe is glass with a protective plastic surround, like with the common hand held probes. It may be possible to remove the plastic and
just use the "naked" glass probe. I'm not sure of the pH range of these controllers, but 6-7 pH should be no problem.
Edit:
Actually, something I tried a few months ago was a new way to vacuum impregnate a carbon gouging rod. The idea was to leave the impervious copper
coating in place and use the rod like a "drinking straw" to "suck up" the impregnating medium. I did this by connecting one end of the rod via PVC
tube to a vacuum pump and dipped the other end in the solution. I tried my linseed oil / siloxane mixture and also epoxy resin diluted about 50:50
with epoxy thinners. The latter mixture sets to a hard rubbery consistancy after a few days.
Unfortunately I couldn't get anything to "suck up", it may have been due to my pathetic vacuum pump (fridge compressor) or maybe it just doesn't work.
Someone with a good vacuum pump might like to give this procedure a try. The diluted epoxy is quite fluid, and I would have thought it would have
worked quite well. When the epoxy has hardened the copper coating can be peeled off to reveal the epoxy reinforced and sealed gouging rod.
Smoking hot melted candle wax was another possibility but it would require the rod to be kept hot to stop the wax hardening as it was "sucked up" the
rod.
[Edited on 14-10-2008 by Xenoid]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I recently picked up one of those very weipro type controllers. You can set the pH control between 3 and 10, I believe. They seem to work all right,
but I haven't setup a new cell yet. The probes are a typical acrylic looking casing around a standard glass probe. I bought an extra probe. If the
probes themselves don't survive, I'll look into a different type that would be more suitable.
Got some stuff together to make a 5 gallon bucket cell, once I get that MMO anode to play with. Going to use a solenoid and gravity to dose the cell
with HCl (probably just muriatic acid) with a yoke or something to restrict flow. Tubing will be PTFE.
Also recently picked up a 5.5v 200A CC/CV power supply, and a 6V 90A CC/CV power supply.
When the MMO anodes get here, I will attempt to plate the MMO coating with LD, I'll let you guys know how that goes.
[Edited on 15-10-2008 by tentacles]
The exact model of controller : http://cgi.ebay.ca/ws/eBayISAPI.dll?ViewItem&item=230289...
The company, gainexpress.biz, lists on their website an industrial pH controller with ATC and a PC interface.
[Edited on 15-10-2008 by tentacles]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
MMO Chlorinator Cell Electrodes
Here's my latest haul from the local Swimming Pool - Spa maintenance centre. Followers of the various anode threads may remember my first MMO
chlorinator electrode assembly which I described in the "Cobalt Oxide Anodes" thread about December, 2007:
http://www.sciencemadness.org/talk/viewthread.php?tid=9572&a...
At the time I left my name and telephone number with the company, so they could contact me if they got any more. Well, they lost my contact details,
but put the used electrodes to one side for me. Well I finally called in again today to see if they had anything, and the attached image shows what I
picked up.
The 5 assemblies in the top row appear to be from the same manufacturer and have 5, 7 and 10 electrodes. The 10 electrode assembly in the top right
location seems to be in very good condition. The small assembly in the lower right location has 5 solid titanium plates, but seems to have lost its
MMO coating.
The electrodes were free, but I very generously gave the guys a $5 donation for their tea fund and trouble. 
They said they would probably have a few more over the coming months. I also called in at another place and they also said they they would put some
aside for me, although this company has promised this in the past and I have yet to see anything from them!
Like "tentacles", I have been thinking for some time of having a go at PbO2 coating an MMO electrode.
EDIT: Upon further inspection it appears that the 5 green assemblies are POOLRITE brand - each top contains 2xM5 and 1xM2.5 titanium bolts - yes, Ti
bolts, they have Ti stamped on the heads. I have never seen Ti bolts before, I wondered why they were not corroded. Externally they are fitted with
heavy brass nuts. There is also a large "O" ring with each top.
[Edited on 19-10-2008 by Xenoid]

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Xenoid: You are definitely a man of immense charm and graces (or was it the five shade clad 'heavys'      (minus the smiles) that came with you to the company premises) for managing to secure that huge haul. (minus the smiles) that came with you to the company premises) for managing to secure that huge haul.
The scene looks more like a photo from DeNora's repair shop that some garage Chlorate maker.
I must try some similar companys around here but swimming pools (wanted swimming pools that is) are a bit thin on the ground.
Cheers,
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by dann2
The scene looks more like a photo from DeNora's repair shop that some garage Chlorate maker.
|
LOL - Good one, Dann2 
Here's a detailed image of one of the POOLRITE 5 electrode units. These are polarised in that they have 3 plain Ti cathodes and 2 MMO coated anodes.
The 10 electrode assembly (mentioned above) is non-polarised and has 10 MMO electrodes. The non-polarised units have the + and - connections
alternated by the controller to prevent build-up of "crud" on the cathodes. The anode assembly has a date code and is 14-12-01. The other units range
in age from 1997 to 2004.
The mesh electrodes are 20cm x 5cm, and are spot welded to Ti straps which in turn are spot welded to the Ti bolts. Near the head of the bolts you can
see a small grey plastic fitting with an "O" ring seal. This small "O" ring assembly fits into a counter-bored insert on the inside of the green lid.
This is a great design, and it makes it very easy to dismantle (versus the epoxy encapsulation method) and modify for home chlorate use. All that is
needed is a new 12-15mm thick PVC lid to suit your cell, counter-bored for the Ti bolt/"O" rings. With a little silicone grease this will provide a
perfect corrosion-free and gas sealed electrical connection. The Ti bolts have a smaller brass nut to hold them in place and the larger brass nut to
provide good electrical contact to the Ti bolt threads.
The small hole to the left side of the lid is for an earth or sensing electrode and was fitted with a small Ti bolt with a 3cm length of MMO wire
attached to it.
One of the POOLRITE 5 element units, (dated 2004) appears to have lost virtually all the black MMO coating from the anodes and is truly worn out (but
still suitable for cathodes or recoating). The other units all have usable MMO electrodes.

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Xenoid
One of the POOLRITE 5 element units, (dated 2004) appears to have lost virtually all the black MMO coating from the anodes and is truly worn out (but
still suitable for cathodes or recoating). The other units all have usable MMO electrodes. |
Careful you do not condem these elements (yet). They may still have a usable MMO coat. I have a piece of corrosion prevention wire anode (MMO on Ti
with Cu core). I put it in a Perchlorate cell (cell containing Chlorate) to see if it would make Perchlorate and let it run for a long long time. The
black coating wore off the wire in places but the wire STILL had an MMO coating of some sort on it as it went on gassing where the black coat was
gone. It just looked like bare Ti but was not. There must have been two coatings of some sort on the wire. Perhaps your anodes are similar.
Try in a cell before assuming the MMO is gone.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I like the looks of those chlorinator elements, especially the plastic spacing brackets. I assume in those devices, they are HDPE or some other
castable plastic, but in a chlorate cell, something similar from PET, PVC, or PTFE would be better.
I'm wondering about the ability of those light Ti straps to carry current. A pool chlorinator doesn't draw nearly as much as we would like to put
through a cell, and with my own systems, I've noticed a minimum of 1" wide or so is necessary when operating at 50+ amps, otherwise, the straps will
heat to the point where they will damage the cell lid or whatever plastic system used to seal them.
If anyone is interested in reworking surplus MMO mesh material, or starting with new, I found that the MMO mesh spot welds beautifully to Ti straps,
which for me was a bit counterintuitive, as Al cannot be spot welded at home, and I thought the Ti would behave like Al in this regard. You can
either buy and rework a cheap Harbor Freight spot welder, or make your own, but it is definitely the way to go.
Tentacles, have you located a useable solenoid valve for the HCl pH control vat? There are varieties that simply pinch the PTFE tubing, and that
might be a good way to go.
[Edited on 24-10-2008 by Swede]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hi Swede
A quick perusal of chlorinator cell specifications, for example here;
http://www.poolstuff.com.au/shop/product_listings.php?Maingr...
indicates they are designed to run from 10 - 50 amps usually as a function of the number of plates. Most 7 plate cells appear to run around 30 - 35
amps. They seem to use a standard strap geometry and fit from 3 to 10 electrodes depending on the application (pool volume, and hence current). The
50 amp cell may use thicker straps, but I don't think so.
The Ti strap on the 5 plate unit I described above has a cross sectional area of about 10mm^2 (10mm x 1mm). This size seems standard on all the
chlorinator units I have seen, even the 10 electrode, so I guess it is OK for at least 30 - 40 amps - may be 50 amps.
BTW - a quick search with Google indicates Ti bolts are fairly readily available, for example in the USA here;
http://www.unitedtitanium.com/boltInventory.html
And in the UK here;
http://www.tibolts.co.uk/
Apparently they are used quite commonly on top range mountain bikes, the UK place ships worldwide and their prices look quite reasonable. I think Ti
bolts have a great place in (per)chlorate cell construction. Having to use SS bolts was a definite drawback with many of my designs as they corrode
badly, especially in the headspace area.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
Anode looks good.
How did you weld it? Is your welder a MIG welder with Ti wire or are you using a
'spot welder' (not too sure what they are universally called). ie. A welder with two Copper electrodes that clamp onto the area to be welded and a
current then passes?
Did you ever try welding Ti to Ti with the spot welder? Perhaps you are having success welding Ti to MMO coated Ti because the MMO coat makes it
possible (guess).
If one was too lazy to weld a Ti strip to the MMO or (God forbid) the welds cause problems you can always simple use larger piece of MMO and take it
out of the cell and connect directly to the MMO outside the cell. A waste of preious MMO real estate but might be the way to go for someone who
can't/won't weld.
The pH controller that has been mentioned is definitely the way to go for pH controll, better that a simple syringe pump. Hope pH probe holds up.
It makes you wonder that if Xenoid had to try his MnO2 anode in a pH controlled cell would it have lasted much much longer than it did? It lasted a
long time in a non controlled cell but if the graph above for Graphite anode erosion has any relation to MnO2 anode erosion, then the MnO2 would go
from being a good anode to a bloody great anode.
Easy to make at the back of a ditch. Not as good as MMO I am sure but then nothing is as good as MMO (if you can get it).
Dann2
|
|
|
| Pages:
1
2
3
4
5
6
..
48 |