| Pages:
1
2
3
4 |
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
What all can be done with NaCl?
NaCl when glowed with boric acid and air is let in at the same time gives off Cl2 (Schulze, J.pr. Ch. [2] 21 [1880] 407).
Metallic Fe reacts with NaCl in a vac. at 800 deg. and above: Fe + 2 NaCl = FeCl2 + 2 Na (Hackspill, Ann. Chim. [10] 5 [1926] 228). KCl, KBr, KI also
react with Fe at 900 to 1000 deg. forming the Fe-halogenide but yielding little potassium (KF gives a bit better yield), both also volatilize at the
same time. Other salts can be reduced to obtain good yield like K2SO4 at 1000 deg. (by-products: Fe2O3, FeS, SO2, O2, after 2hr heating, 80% yield of
K, larger filings of Fe can be used). K2CO3 and Fe is an explosion risk since CO is a by-product, this can combine with K to form explosive compounds
(so the same thing when reducing K2CO3 with C). See Gmelin.
Melting together S with NaCl forms sodium sulfide and SCl2 (DE49628). This is a bit more complicated, the patent has a schematic where molten sulfur
acts on molten alkali chloride, where then both of those mentioned compounds form at the same time. They stress temp. needs to be high enough
otherwise you get sulfide and Cl2. The vapor SCl2 is also contaminated with S. For which reason they recommend large excess of alkali chloride.
Cr2O3 or Mn2O3 heated to red glow with NaCl, causes them to decompose when air or O2 is lead into it, forming Cl2, and with water vapor - HCl
evolution (Hargreaves, Robinson, E.P. 508 [872]; Ber. 5. [1872] 1064).
Leading steam into a melt of NaCl and silicate, gives the reaction: 2 NaCl + H2O + Na2SiO3 = 2 HCl + Na4SiO4 (DE 382216 [1916]).
The outcome of the action of NaCl on SiO2 depends on the atmosphere, in which the mixture of both is heated according to:
a) 4 x NaCl + y SiO2 + x O2 = 2 x Na2O, y SiO2 + 2 x Cl
b) 2 x NaCl + y SiO2 + x H2O = x Na2O, y SiO2 + 2 x HCl
c) 4 HCl + O2 = 2 H2O + 2 Cl2
In a stream of dry air, only reaction a occurs. In moist air, a, b, and c occur together. In moist nitrogen, only b occurs. In moist air, reaction b
is predominant at up to 1000 deg. the extent of the action is small however, but at 600 deg. the reaction becomes noticeable. Increasing the
temperature and moisture content increases the extent of the reaction. The deciding factor is the size of the contact surface of the reacting material
(Gmelin, Na, Sys. 21, 329).
With water: overheated water vapor will react with NaCl to form NaOH and HCl. The decomposition through water comes to a stop when for 8 moles NaCl, 1
mole NaOH has formed. If water vapor is led over NaCl in a heated platinum crucible, a considerable reaction occurs under 500 deg. The reaction starts
clearly around 700 deg., the speed of which increases with the temp. In a porcelain crucible the decomposition isn't quantitative (Gmelin).
Speaking of which, before NaCl becomes heavily restricted, where can one get or extract it? Besides the ocean and salt flats, which might be only
ready useful sources if you are near enough.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I would think that NaCl, along with KCl, MgSO4, CaSO4, Ca(HCO3)2, and various minor solutes in sea water such as NaF, LiCl, RbCl, CsCl, etc., would
become "restricted" or "controlled" chemicals at about the same time as H2O does similarly. This is in view of the fact that most of the world's
water is sea-water, containing these in solution. People will be made to pay a huge amount of dollars every time they want a drink of water, which
will have an excise tax imposed on it. Access to rivers, lakes, and the sea will become strictly controlled by huge armies of cops, to make sure that
no-one helps themselves to free supplies. Rainwater collection will be prohibited.
But I think that other household chemicals, such as starch, cellulose, sucrose, C2H5OH, CH3OH, iso-C3H7OH, NaOCl, Ca(OCl)2, NaHCO3, Na2CO3, citric
acid, and tartaric acid, will become "restricted" or "controlled", and subject to huge excise taxes like that already on C2H5OH, well before NaCl and
H2O do. Especially the organic substances, because they can be either nitrated to make explosives, or fermented and distilled to make C2H5OH.
[Edited on 25-5-09 by JohnWW]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The Totalitarians are, as we speak, looking with suspicion at air as a source of precursors like O2 (potent oxidiser), nitrogen (NH3) among others,
and various acidic oxides.
Large sections of society could be gainfully employed in restricting public access to this ubiquitously dangerous mixture of substances.
Wholesale metering of air, to determine personal usage would be perceived to progress the WOD/WOT alliance.
Known chemophiliacs could be monitored for their lifestyles and their stepping out of line could result in a stint in Gitmo or Australia (once it's
depopulated to absorb the influx). . .
Australia here we come!
|
|
|
littlepop
Harmless

Posts: 8
Registered: 27-5-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The_Davster  | Methyl salicilate can be gotten from the wintergreen plant, which with base can be converted to salicylic acid. [rquote]
Willow bark is where the first salicylic acid came from. I don't know the concentration, but it does help a headache.
[Edited on 5-27-2009 by littlepop] |
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Black poplar (Populus nigra L.) leaves contain salicin. Willow tree bark is rich in salicin. Wood sorrel (Oxalis acetosella L.) leaves contain oxalic
acid, or also in the mashed leaves of Sorrel (Rumex acetosa L.). Oxalic acid exists in quite a few plants, generally not as a free acid though. The
Benzoin Tree (Styrax benzoin) bark is a source of benzoic acid. Mannite in the sweet exude from the stem of Fraxinus Ornus and Fr. rotundfolia. Sodium
carbonate from the burned plants near the ocean, potash from land plant ashes. Although plants can be a source of several basic chemical compounds,
these are usually more economical to produce by other means (energy costs and isolation from other products). Books like:
The Organic Constituents of Plants and Vegetable Substances and Their Chemical Analysis
http://books.google.com/books?id=wRgAAAAAQAAJ
An Introduction to the Chemistry of Plant Products
http://books.google.com/books?id=nY8HAQAAIAAJ
have more of that kind of information, and if you dig hard enough, you might find more specific analytical and extraction procedures.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I just aquired the 1927 edition of "Laboratory Experiments in Organic Chemistry," by Roger Adams and J. R. Johnson. It's mostly a repeat of
procedures I already have but there are some interesting ones new to me:
1. This is a first year, first semester book but the student gets to make n-valeronitrile using 39g of "powdered sodium cyanide (handle with
great care)."
2. Syntheses of fluorescein and eosin dyes.
3. and my favorite: "Hippuric Acid from Urine." The procedure says "before retiring at night, ingest 5g of pure sodium benzoate (or ammonium
benzoate)..." "Collect the overnight urine voided the next morning and isolate the hippuric acid as described below."
http://en.wikipedia.org/wiki/Hippuric_acid
Those old time chemists really knew how to party. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chloric1
International Hazard
    
Posts: 1141
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Quote: Originally posted by Formatik  | | Quote: | | Originally posted by Liedenfrost Do you think the production of Carbolic acid from the distillation of Salicylic acid and Calcium oxide is
practical, that is, do you think it might rival the coal process for small scale manufacture? |
This is the first time I've heard of CaO and salicylic acid. Beil. II 952 mentions that heating salicylic acid with 3 or more moles KOH to 250 deg.
doesn’t change the acid. With 4 moles KOH there is at 300 deg. a partial decomposition of salicylic acid to CO2 and phenol, but with 6 moles KOH the
acid remains unchanged at even 300 deg. (Ost, J.pr. [2] 11, 392). Heating 1 mol salicylic acid with 6-7 moles NaOH to 300 deg. decomposes most of the
acid into CO2 and phenol, using 4 moles NaOH the decomposition is nearly complete. However, with 8 moles NaOH most of the acid remains unchanged
(Ost). Distilling calcium salicylate Ca(C7H5O3)2, gives besides phenol in the distillate, also small amounts diphenyl oxide (Goldschmiedt, Herzig, M.
3, 133). I don’t know how these compare to the coal process. |
I read almost 20 years ago, probably in Merck index, that salicylic acid is decomposed to phenol by heating. I remember heating it and maybe a third
or half sublimed on the sides of the container unchanged and the other portion formed a clear liquid that smelled strongly of phenol . I did not analyze so the experiment needs repeating. If I do not order salicylic
acid then I will have to buy some generic aspirins and extract the salicylic acid. Note the ASA is easily deacetyled but boiling with 10% H2SO4 or so
I read. . I did not analyze so the experiment needs repeating. If I do not order salicylic
acid then I will have to buy some generic aspirins and extract the salicylic acid. Note the ASA is easily deacetyled but boiling with 10% H2SO4 or so
I read.
Fellow molecular manipulator
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Preparation of phenol by heating salicylic acid and CaO was one of the experiments in the chemistry sets from the 1950's.
Now they'd arrest you for putting those two chemicals in a "toy".
|
|
|
chloric1
International Hazard
    
Posts: 1141
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Quote: Originally posted by entropy51  | Preparation of phenol by heating salicylic acid and CaO was one of the experiments in the chemistry sets from the 1950's.
Now they'd arrest you for putting those two chemicals in a "toy". |
A hence freaks like me are hydrolysizing powdered aspirins with battery acid

Fellow molecular manipulator
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Yes, I think amateur chemists born after 1970 are definitely at a disadvantage.
Probably at least half my chem inventory was bought down at the drugstore in the 1960's. And cheaply too!
[Edited on 1-10-2009 by entropy51]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by chloric1  | Quote: Originally posted by entropy51  | Preparation of phenol by heating salicylic acid and CaO was one of the experiments in the chemistry sets from the 1950's.
Now they'd arrest you for putting those two chemicals in a "toy". |
A hence freaks like me are hydrolysizing powdered aspirins with battery acid
|
I prefer to use base, as the hydrolysis is very rapid and homogenous. After fairly brief heating, acidify, filter, and recrystallize from boiling
water. I did the recrystallization in a metal bowl once, and the liquid that sloshed up the sides got significantly hotter and charred....it reeked of
phenol.
"3. and my favorite: "Hippuric Acid from Urine." The procedure says "before retiring at night, ingest 5g of pure sodium benzoate (or ammonium
benzoate)..." "Collect the overnight urine voided the next morning and isolate the hippuric acid as described below.""
Is it wrong that I want to try this?
[Edited on 9-30-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
chloric1
International Hazard
    
Posts: 1141
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Well the hydrolysis of asprin with diluted H2SO4 was to isolate the salicylic acid. The sulfuric acid is a catalyst to break off acetic acid from the
aspirin. It can then be heated with CaO. I would presume none of the salicylic acid will sublime out . .
@entropy- Yes I have ALWAYS said that being born after 1970 that I missed out! I was even saying that in the late 1980's when I first started my
chemistry explorations. The positive side of this is since the dawn of my hobby I have been looking on how to either buy OTC reagents with out fuss
or how to make my own. The stepping up of restrictions during the 1990's was my basic training so to speak. Thats good becuase these days even the
very early 1990's seem like a golden era compared to today. Where are going to get a gallon of CCl4 for $20? Or better was a kilogram of PCl5 for
$50!! In 1992 this was what was availabe from Chem-Lab supplies.
Wow I totally deraled this thread Sorry! BTW does the phenol distill from
the CaCO3 residue or do you just lixivate with water or alcohol? Sorry! BTW does the phenol distill from
the CaCO3 residue or do you just lixivate with water or alcohol?
Fellow molecular manipulator
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by UnintentionalChaos  | "3. and my favorite: "Hippuric Acid from Urine." The procedure says "before retiring at night, ingest 5g of pure sodium benzoate (or ammonium
benzoate)..." "Collect the overnight urine voided the next morning and isolate the hippuric acid as described below.""
Is it wrong that I want to try this? |
No, I thought that one looked totally great. When you publish pictures
of your experiment, though,please omit those of urine collection.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | Is it wrong that I want to try this? |
If you use food grade Na benzoate and have normal kidney function it
won't be wrong at all. We'd love to hear about it!
Actually I think eating cranberries might have the same effect, IIRC they contain lots of hippuric acid.
@chloric1, if I were to try to collect the phenol I would probably try steam distilling it out. Phenol does steam distill! You would need to need to
acidify it before steam distilling, of course.
[Edited on 1-10-2009 by entropy51]
[Edited on 1-10-2009 by entropy51]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
@chloric1: nice to see you using the archaic term "lixivate." 
| Quote: |
Is it wrong that I want to try this?
|
No, go for it. I feel the human body is an underutilized bioreactor for home chemistry. (Just kidding)
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | I just aquired the 1927 edition of "Laboratory Experiments in Organic Chemistry," by Roger Adams and J. R. Johnson. (cut) |
That is the First Edition of the book. It went through at least seven editions, the last appearing to be the Seventh in 1979, after
which the authors died.
See http://www.orgsyn.org/obits/johnsonjr.pdf
for the obituary of Prof J R Johnson, 9 Aug. 1900 - 25 May 1983. No edition of the book appears to have ever been scanned and uploaded.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by entropy51  | | Quote: | | Is it wrong that I want to try this? |
If you use food grade Na benzoate and have normal kidney function it
won't be wrong at all. We'd love to hear about it!
Actually I think eating cranberries might have the same effect, IIRC they contain lots of hippuric acid.
@chloric1, if I were to try to collect the phenol I would probably try steam distilling it out. Phenol does steam distill!
[Edited on 1-10-2009 by entropy51] |
Well, the heating with CaO (Ca(OH)2?) is what achieves decarboxylation. At those temperatures, I suspect it might distill from the mix, much like
benzene does from the fusion of benzoate and NaOH. Either that, or it remains trapped as calcium phenoxide. In that case, I would add water and steam
distill.
Would you mind listing the extraction procedure, Magpie?
[Edited on 10-1-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The first of these books, which has these publication details:
Author: Wittstein, Georg Christoph, 1810-1867; Müller, Ferdinand von, 1826-1896, tr
Publisher: Melbourne, MʼCarron, Bird & Co., 1878
can be downloaded from http://www.archive.org/details/organicconstitu01wittgoog ; the full URLs are:
http://ia351402.us.archive.org/0/items/organicconstitu01witt... 20.2 Mb and
http://ia351402.us.archive.org/0/items/organicconstitu01witt... 12.8 Mb
The second one, which has these publication details:
Author: Haas, Paul, 1877-; Hill, Thomas George, 1876-
Volume: 1
Subject: Botanical chemistry
Publisher: London, New York, Longmans, Green, 1928; and
Author: Haas, Paul, 1877-
Volume: 2
Publisher: London ; New York ; Toronto : Longman's, Green and co, 1929
can be downloaded from:
http://www.archive.org/details/introductiontoch01haas
http://www.archive.org/details/introductiontoch02haas .
The full URLs are:
http://ia311036.us.archive.org/0/items/introductiontoch01haa... 21.7 Mb and
http://ia311036.us.archive.org/0/items/introductiontoch01haa... 10.2 Mb and
http://ia311009.us.archive.org/3/items/introductiontoch02haa... 10.3 Mb and
http://ia311009.us.archive.org/3/items/introductiontoch02haa... 5.1 Mb
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
| Quote: |
Would you mind listing the extraction procedure, Magpie?
|
Here you go:
"Measure the volume of urine and for every 100 cc. add 25 g. of pulverized ammonium sulfate and 1.5 cc. of cond. sulfuric acid. Stir thoroughly until
the ammonium sulfate is dissolved, and set aside for several hours in a cool place or in an ice-bath, until the hippurcic acid has separated as
completely as possible. It is advantageous to allow the solution to stand in a cool place overnight, since the hippurcic acid separates slowly.
Filter the crystals with suction and wash with a little ice-water. Transfer the crystals to a beaker and purify by crystallization from about 50 cc.
of hot water, with addition of 1-2 g. of decolorizing charcoal. To aid in the removal of colored impurities it is advantageous to boil the hot
solution for about 10 minutes with decolorizing charcoal before filtering the solution. Allow the solution to stand for at least an hour, and cool in
an ice-bath before filtering the crystals of purified hippurcic acid. Calculate the percentage yield of the hippurcic acid isolated (assuming a
complete conversion of the ingested benzoate into hippurcic acid)."
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
another way to Cl2
I thought there was all that was to its basic thermal decomposition. But some time ago I reading Darstellung von Chlor- und Salzsäure by
Nikodem Caro it was giving some procedures and citing DE51084, which states if MgCl2.6H2O is heated in an airstream at a temperature not exceeding 120
deg., then the chloride hydrate can be made up to 80% waterfree without it melting, nor that HCl or Cl2 is lost. Then in this condition it can be
subjected to higher temps. without melting. And then by further heating nearly all of the water can be driven off. But since in the first heating
phase one heats very close to the melting point, the first dehydration phase is a careful operation.
Then the patent states to make it go smoother what they do is take a hot MgCl2.6H2O solution, a given amount of e.g. 50% of anhydrous MgCl2 is added.
By cooling you get a solid mass which is broken into pieces. The latter can be heated in a continuous apparatus to 300 to 400 deg. without melting. At
this temp. the pieces are subjected to an airstream (dried by H2SO4, CaCl2, etc.) to drive off water, and so on. Eventually the anhydrous MgCl2 is
subjected to fire and thus liquefied at red glow and then subjected to air stream, Cl2 is given off and MgO also forms. Rest is described in cited
patent.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Crazy. But I suppose it makes sense: MgO has such an insanely high HoF, it's highly likely to form as a result of any possible reaction, at least
given enough delta T.
Tim
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
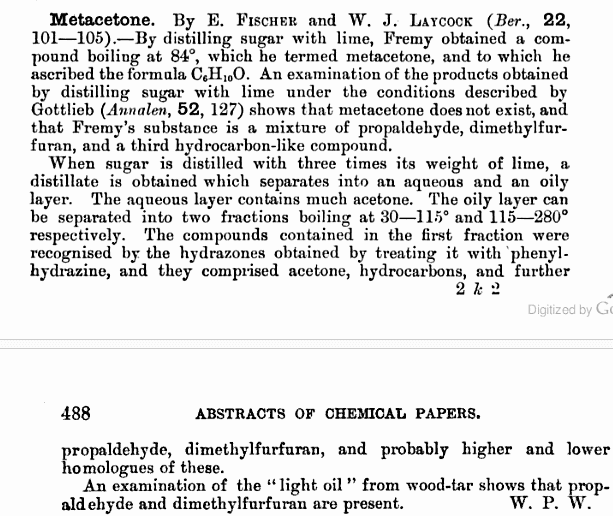
Acetone forms by the dry distillation of citric acid (Robiquet, Berz. Jahresber. 18, 502 and Ann. 25, 138), and by distillation of sugar, starch, or
gum with CaO (Fremy, Ann. 15 [1835], 279). Fremy's descriptions of the distillations are given below. And the part of the paper dealing with it is
also attached below.
Basically:
* The sugar and lime need to be very fine and they both must be as extremely intimately mixed as possible. The smallest particle of sugar should react
with the smallest part of the lime. Because the unreacted sugar particles decompose and yield a volatile oil with a burnt sugar odor that normally
forms by regular distillation.
* The ratio of 1 part sugar and 8 parts lime has proven to be the best. At least 500g of sugar is recommended.
* The mixture is then distilled in an apparatus which has to be at least as double as large as the mixture since the volume increases and the mixture
rises. At this point the flame is removed. The reaction will continue on its own until it stops by itself. If the mixture was intimate enough, only
little flammable gas is evolved (don't do it in an enclosed area or heat too strong) and an oily substance of varying composition distills over. It
has an etheral odor and amber color.
* The oil shaken with water causes it to partly solubilize. The water soluble portion heated in a water bath at 70-80 C, is where acetone distills
over. To get the acetone pure (Bp. 56 C), it needs to be rectified several times in the water bath.
* The oil which remains insoluble when shaken with water is obtained pure by rectification. At first acetone comes over, later the pure oily compound
goes over. To be obtained entirely pure, shake again with water, rectify, and leave it in contact with CaCl2 to remove all water. The oily compound is
colorless when pure and has a pleasant odor, is soluble in alcohol and ether, insoluble in water. It boils by 84 C. The formula determined for this
was C6H10O. The material is called "metaceton". I'm not sure what exactly that is. I've looked through Beilstein a bit but found no compound with that
formula and boiling point.
* The same said of the distillation with sugar, is said to apply for distillation using starch or gum instead, with the same compounds forming. Starch
forms more "metaceton" than acetone. And gum forms a lot more acetone than metaceton.
* The distillation likely gets messy, so it's best to use unloved apparatuses. The yield of acetone probably sucks as not all sugar is converted to
acetone some to metaceton and gases, but still an interesting method of formation.
Attachment: Ann. 15, 279.pdf (389kB)
This file has been downloaded 646 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
you can't find it because it doesn't exist
[Edited on 28-3-2010 by not_important]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I thought there was something fishy about that determination. The boiling point and formula found for acetone were pretty spot on though. Thanks.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Propaldehyde is useful, reduce it to n-propanol or oxidisr to propionic acid. And 'dimethylfurfuran' may be http://en.wikipedia.org/wiki/2,5-Dimethylfuran
|
|
|
| Pages:
1
2
3
4 |