| Pages:
1
2
3
4
5
6
..
10 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Doh !
And i was so close to winning ...
I suppose the fact that they're paracetemol tablets may have been discovered sooner or later.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
You should try that extraction. The extractions and synths will most likely come out separate
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You didn't even get your hands dirty!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Work Smarter not Harder.
That's my motto.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by aga  | Doh !
And i was so close to winning ...
I suppose the fact that they're paracetemol tablets may have been discovered sooner or later. |
Made
me laugh.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice synths. I wouldn't worry about dynamics changing too much, the basic idea (No pun intended) is pretty cool. Antimony hexacyanoferrate(II?) was
pretty cool!
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
I also don't agree with the possibility of having a "longest streak" prize. My personal entry, a compound with a MW of 23000+, takes several days to
recrystallise, so of course I cannot submit it until then.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quick, someone make this one.
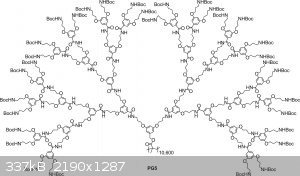
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Afraid not. That's a polymer with n=10000. Mine is a discrete molecule, 10 points for guessing what it is!
Edit: at least one person has already
[Edited on 18-11-2014 by Oscilllator]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Psht, no. It's a discrete molecule with exactly 10,600 units, didn't you read the diagram? 
I confess I've no idea what compound you're getting at, though I'm looking forward to seeing the results. (Are you biosynthesizing enzymes?)
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Nono. Proteins don't count, remember? Big hint: This is an inorganic compound
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Didn't know whether you were going to argue deliberate biosynthesis was a qualifier.
Not a clue.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Phosphotungstic acid has been mentioned, but that doesn't have a MW of 23000+.
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
I am going way to far to counter this.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yeah, in UNDER 24 h, so you can submit fast! 
But what is it? It strikes me as neither a polymer (an oligomer maybe) nor a protein.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Talking about this 'antimony ferrocyanide':
Quote: Originally posted by bismuthate  | Antimony Ferrocyanide
Sb4(Fe(CN)6)3*(H2O)25
1573.2702 g/mol
5g of antimony from a nearby welding supply was added to 40mL of 39% muriatic pool acid.
It is left to react for 2 days.
Decant the solution.
Mix this solution with 6g of potassium ferricyanide obtained from impure blue toner and 6g of sodium metabisulfite in 40mL of water.
3K4[Fe(CN)6]+4SbCl3=Sb4[Fe(CN)6]3+12KCl
Filter and dry the precipitate.
https://www.dropbox.com/sc/87jprnifb6tdpud/AACuptOR9fzKQCR7k...
[Edited on 16-11-2014 by bismuthate] |
I have serious doubts that that is what Bismuthate obtained.
Antimony is an acid former, not a base former. Sb(III) salts are almost unheard of: even SbF<sub>3</sub> in the molten state conducts
electricity only poorly.
SbCl<sub>3</sub> is a prevalently covalent compound that hydrolyses very strongly in water.
Salts of antimony invariably contain the element as anions, like antimonites, antimonates or halo substituted forms of these. H2SbF7 is a 'superacid'.
Bismuthate should analyse his compound for antimony, before making these claims.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
If I may make a suggestion for future contests, perhaps make it a rule that contestants work from a referenced procedure that they report in
the write-up and that the compound is characterised in that or another reported reference and that there is at least some cursory attempt to correlate
the synthesised product to what it should be (be it m.p or appearance of crystals).
Simply growing decent crystals of the compound you made can go a long way to convincing sceptics that what you have is (a)reasonably pure and (b)what
you claim... but only if compared (at the very least) to what the paper describes the crystals should look like.
Otherwise, I fear that this contest will be riddled with, "I don't believe you"'s.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Part of the rules state that the procedure must be described. I see no reason to limit the allowed procedures to those already referenced.
I would like to see more proof that antimony ferricyanide was formed though, for the reasons blogfast25 mentioned.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by blogfast25  |
Yeah, in UNDER 24 h, so you can submit fast! 
But what is it? It strikes me as neither a polymer (an oligomer maybe) nor a protein. |
It was from an attempt to build 3-dimensional, structured polymers similar in complexity to biomolecules. http://phys.org/news/2011-01-giant-molecule.html
The original paper is in German, I think. I do not have access. doi:10.1002/ange.201005164
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It's one of these I think.
http://en.wikipedia.org/wiki/Dendrimer
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
blogfast25, Sb3+ salts are actually quite common, but I'll analyze it.
I can analyze it for ferrocyanide, but how would I do so for Sb is a challenge.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by bismuthate  | blogfast25, Sb3+ salts are actually quite common, but I'll analyze it.
I can analyze it for ferrocyanide, but how would I do so for Sb is a challenge.
|
Are you sure the antimony was pure? Look for zinc impurities, maybe? Just a guess.
If it is pure, it's containing of Ferrocyanide should be enough.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Copper plates out antimony, like it does arsenic. So a copper plate or wire in the solution of your product should go black quickly (I've done this
with Sb(V) and it was very quick). Cu will not plate out any Zn or Sn which may accompany it.
The test is a preliminary forensic screening test for arsenic (still used today). Sb and As are distinguished from each other by the latter's deposit
dissolving in hypochlorite solutions.
@ES and Union: thanks! Interesting stuff...
[Edited on 18-11-2014 by blogfast25]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Now normally I would do that but I lack NaOH and therefor a way to put the Sb into solution with certainty.
EDIT: well actually I would use a marsh test, but that wouldn't work for obvious reasons.
[Edited on 18-11-2014 by bismuthate]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Yes, that's the word. Dendronized polymer, though.
|
|
|
| Pages:
1
2
3
4
5
6
..
10 |