| Pages:
1
2
3
4
5
6
..
77 |
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Unfortunately, I wasn't planning on doing any additional videos unless I get a new camera. The sound is shot and I can't use an external mic with it.
I get so much more done without constantly editing and recording, too...
I did do a writeup with some pictures in prepub though, which I suggest you have a look at.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

Making some water free KOH by melting and boiling it out in a silver pot. It is ready when it stops boiling.
Care should be taken while working with it, especially when it's molten 
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Kristof, your pictures are amazing. What were you trying to make?
I'll try to compete with my more modest photos:
1. Terbium, after a real beating. It's a lot shinier on the inside. Wikipedia says it's soft, but doesn't say it's ridiculously durable.
2. Bismuth stairs.
 
Edit: Kristof, I was referring to your post with the hydrogen apparatus.
[Edited on 2-12-2013 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
For the hydrogenation we tried to make a 1,1 disubstitued hydrazine from a nitroso coumpound (both quite toxic...). Sadly the N-N bond broke and I
got back the disubstitued amine and some ammonia.
For the molten KOH, we needed something to distill from ethylamine, since we opened a few cylinders of it, what were stored for a few years. It was
great to distill 2-5 liters of this gas, everything smelled like fish, even since it was under a fume hood 
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
A very all day compound: K2SO4
  
The individual crystals making up this cluster are about 1 cm long.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by kristofvagyok  | 
Making some water free KOH by melting and boiling it out in a silver pot. It is ready when it stops boiling.
Care should be taken while working with it, especially when it's molten 
|
definitely would be scared to do this thing... but anyway, very interesting 
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|

some beautiful uranophane crystals posted by kristofvagyok in the uranium thead.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
m-dinitrobenzene
 
smells a bit like almonds, but more like marzipan. in the spirit of Christmas
all above information is intellectual property of Pyro.  |
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
A biphasic highly fluorescent oil under UV light, containing some substitued coumarine what I made a few day ago.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
One of your best photos, kristof. 
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
New element sample  Probably only a few mg Probably only a few mg

Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Tried the oxidation of paracetamol to p-benzoquinone with aqua regia. The appearance looks good but this afternoons melting point will give a better
idea of how the reaction turned out, looks nice though 

|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Not chemistry related, but pretty nonetheless. The panorama feature on my camera is useful for capturing such phenomena.

I wish I could upload the monster 2800x1440 full size photo.
Bonus: Is it a solar halo, or a street lamp at 1.21 gigawatts?

At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Hydrolysis of ASA to yield SA gave some nice crystals: ( 95% yield too  ) )

Some ethyl acetate from non-acetone nail polish remover.

[Edited on 14-12-2013 by HeYBrO]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
@Mailinmypocket: Looks really good. It appears as if you sublimed it? I've noticed that no mater how clean you make it, it eventually gets a green
tinge and it can only get worse from there. Use it as soon as possible! Maybe storing under argon would help...
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
@DJF90 - Thanks, not sublimed though. These crystals were produced by 2x recrystallization from boiling 90% 2-propanol. I plan on sublimating them
before taking a mp though. After the initial reaction with aqua regia, quenching with water, and filtration I noticed small black bits in the filter
cake.
When dissolved in boiling alcohol the first time it yielded an extremely deep red (almost black) solution. I think there may have been some over
oxidation or likely a bit of chlorination happening during the reaction, despite being kept in ice, which yielded side products. Anyhow, left to cool
slowly with insulation long orangey-yellow needle like crystals formed from the wine red liquor which were again, recrystallized from boiling
2-propanol. That is what produced the crystals seen above. I took your suggestion and flushed the bottle with argon in the meantime until a
sublimation and mp can be done though.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Bismuth trioxide thermite, a series of explosions that happened in a short amount of time
   
|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
I don't have the best camera. I was inspired by other photo's, so I thought let's try it myself.
<img class="decoded" src="http://www.sciencemadness.org/talk/files.php?pid=311781&aid=28118"
alt="http://www.sciencemadness.org/talk/files.php?pid=311781&aid=28118"></img>

[Edited on 19-12-2013 by DutchChemistryBox]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Very pretty. What is it?
Best to include a description of your experiment along with the photos, as others have done!
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
GFP expressing E. coli
Not the best quality picture ever, but showing clear fluorescence though.
This is a 50 ml tube with a pellet of E. coli expressing green fluorescent protein (GFP) above a UV lamp. The picture is taken through a filter,
filtering the UV/blue light, but not the green light.

|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
Some pretty pictures
They are all empty, LOOOOOOONG ago.
Attachment: Vintage Pharmaceuticals.rar (2MB)
This file has been downloaded 1171 times
|
|
|
bfesser
|
Threads Merged
19-12-2013 at 14:22 |
thebean
Hazard to Others
  
Posts: 116
Registered: 26-9-2013
Location: Minnesota
Member Is Offline
Mood: Deprotonated
|
|
Quote: Originally posted by Mailinmypocket  | Tried the oxidation of paracetamol to p-benzoquinone with aqua regia. The appearance looks good but this afternoons melting point will give a better
idea of how the reaction turned out, looks nice though 
|
Would you care to right your procedure up? I'd like to try it.
"You need a little bit of insanity to do great things."
-Henry Rollins
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Sure! I used the procedure posted by our own benzylchloride1 in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=8250
I still have the stuff stored under argon waiting for a chance to take a melting point, when time permits... Damned holidays
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Here is an old photo I found when I stained a nematode with methylene blue (which stains acid regions like proteins and nucleic acids)
Notice the flagella which help nematodes move.
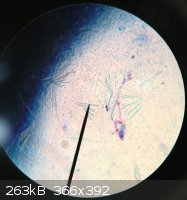
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Some trimethylsilyl acetylene’s silver salt just when it was ignited.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
| Pages:
1
2
3
4
5
6
..
77 |