| Pages:
1
2
3
4
5
6 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I would read up on the dangers of As and As2O3 and would keep the rock under lock and key (display cabinet), so no humans or other animals can get
their paws on it.
[Edited on 6-1-2014 by blogfast25]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I've gotten powder of the stuff on my fingers due to carelessness, and experienced no symptoms. Still, with arsenic, you can never be too careful.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | I've gotten powder of the stuff on my fingers due to carelessness, and experienced no symptoms. Still, with arsenic, you can never be too careful.
|
I must say, I would probably be defecating myself after such an experience 
I would think however, that if Skutterudite was largely toxic there would be a considerable amount more literature to found online regarding such
toxicity and relating concerns. Perhaps the minerals crystal structure itself helps to reduce the Arsenic toxicity by 'isolating' it within its
crystal structure.
Wikipedia states "The unit cell can be considered to consist of eight smaller cubes made up of the Co atoms. Six of these cubes are filled with
(almost) square planar rings of As, each of which is oriented parallel to one of the unit cell edges.... The As atoms then form octahedra with Co in
the centre." So perhaps the outside structure of Co, Fe, or Ni helps isolate the As. I added some 'commentary' to the Wikipedia picture, as I think it
may help to explain the structure of Skutterudite somewhat better, see below.
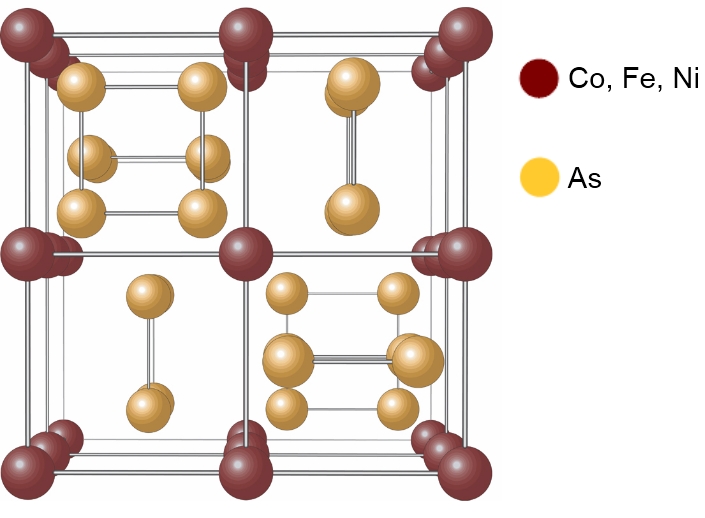
Please note that this diagram is just my interpretation of the above statement, and please correct me if it is wrong, as I am not a mineralogist by
any means.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm sure its low solubility keeps its toxicity low (although I wouldn't want to leave it sitting on my hands for any length of time).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by BobD1001  | | I would think however, that if Skutterudite was largely toxic there would be a considerable amount more literature to found online regarding such
toxicity and relating concerns. |
There is a huge amount of literature online regarding this toxicity, but
most is not specific to just one arsenic-bearing mineral. See: <a href="https://en.wikipedia.org/wiki/Arsenic_contamination_of_groundwater"
target="_blank">Arsenic contamination of groundwater</a> <img src="../scipics/_wiki.png" />
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by elementcollector1  | I've gotten powder of the stuff on my fingers due to carelessness, and experienced no symptoms. Still, with arsenic, you can never be too careful.
|
Wow, EC1, do wear latex gloves at a minimum! 
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  | | There is a huge amount of literature online regarding this toxicity, but most is not specific to just one arsenic-bearing mineral. See: <a
href="https://en.wikipedia.org/wiki/Arsenic_contamination_of_groundwater" target="_blank">Arsenic contamination of groundwater</a> <img
src="../scipics/_wiki.png" /> |
Bfesser, I fail to see where in this link it describes the toxicity of Skutterudite. Of course Arsenic's toxic properties are hugely documented,
however, I was specifically referring to this mineral, for which there appears to be a lack of toxicological information.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
It doesn't describe the toxicity of skutterudite. As I said, most of the published information is not specific to a particular arsenic-bearing
mineral, because in the environment, it rarely matters much which mineral species provide the source for the groundwater contamination, only that they
do. Why do you require toxicological information for one particular mineral species? For most minerals, it doesn't make sense to gather such data.
Also, a mineral species can contain various impurities and can vary slightly in composition between localities and even specimens.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Update: Took another gram or so and put it in a test tube, filling the test tube up with sulfuric acid and a little water. So far, it's exhibiting the
exact same reaction as with the bleach - minor bubbling, some black powder that easily forms a suspension. I am so confused. This shouldn't be cobalt
oxide - that should be in solution as cobalt sulfate.
Maybe I do have to try 'cracking' this with sodium nitrate and sodium hydroxide?
Update for the bleach tube: The cobalt oxide is now much larger-grained, coming out of suspension very quickly. All but the coarsest of the
skutterudite particles seem to have dissolved - it's hard to tell by color which is CoO and which is skutterudite, but I'm fairly certain.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
2 months later, and not much has changed on the test tube scale - in either case. I'm going to see if the ore responds in any way to a blowtorch (in
really small quantities!). If yes, I guess I should try sublimation under vacuum...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No joy with the bleach extraction?
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Nope. No change at all...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Ok, so I thought up a few possible reactions. Sorry if they don't work.
1A skutterudite+HCl+Na2S2O3
1B skutterudite+H2O2+Na2S2O3
One of these, If they react as I predict, would yield As2S3.
2 skutterudite+HCl+I2
should yield arsenous acid
3 skutterudite+H2O2+HCl
Again this should yield arsenous acid.
Again sorry if I mde mistakes.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
H2O2 and HCl is something to try, but keep in mind that this stuff was nigh-immune to concentrated sulfuric acid. Also, what happens to the cobalt in
each of these cases?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
It theoreticaly would form cobalt chloride in solution.
[Edited on 19-4-2014 by bismuthate]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I wonder if fusing and quenching may help. It's often used on minerals to render them responsive to strong acids, based or other reagents.
By strongly heating (melting, if possible), followed by quenching in ice cold water, the crystalline structure is often disrupted and becomes more
'glassy'. The glassy structure can then be ground up and tends to be more responsive to chemical attack.
Worth trying on a tiny piece, IMHO...
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by blogfast25  | I wonder if fusing and quenching may help. It's often used on minerals to render them responsive to strong acids, based or other reagents.
By strongly heating (melting, if possible), followed by quenching in ice cold water, the crystalline structure is often disrupted and becomes more
'glassy'. The glassy structure can then be ground up and tends to be more responsive to chemical attack.
Worth trying on a tiny piece, IMHO... |
Unfortunately, all of my stock is a fairly fine powder at this point. Also, wouldn't we want to avoid formation of gaseous arsenic or arsenic oxide
under uncontrolled conditions?
Per the cobalt: Is there some complexing agent that can separate it and arsenic?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The mystery is that cobalt ad arsenic would normally be sooo easy to separate. So there's something about the ore's unusual structure that's making
things hard.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Found this tidbit from... answers.com?
| Quote: | Skutterudite fuses on charcoal, giving an arsenic (garlic) odor and forming magnetic ball. A cobalt-rich skutterudite ball dissolves in nitric acid to
form a pink solution, though iron and nickel are usually present in sufficient abundance to mask the color. Tests on analyzed samples showed that a
nitric acid solution of (cobalt) skutterudite neutralized by ammonia becomes red-violet and a red-violet precipitate settles out. Nickel skutterudite
under the same conditions gave a blue-violet solution and a pale green precipitate. Rarer ferroan skutterudite gives a strong brown iron-hydroxide
precipitate that will mask either of the other elements.
|
So... I guess I need some nitric acid then.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Per the post above, I wonder what happened to the arsenic?
Ah well, time to test with a few drops of nitric acid. Going to dilute it, so I don't blow my face off...
EDIT: I was so very wrong about how much to dilute it. The test tube overflowed almost immediately! (I quickly washed my hands, hope for the best!) On
the plus side, the supernatant liquid is now a ruddy brown (indicating both presence of iron and a healthy reaction), and there is a fluffly gray
precipitate that is so far insoluble (though more nitric acid should determine this). If this precipitate is elemental arsenic, I will be a very happy
man.
[Edited on 5-3-2014 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
EC1:
Your insoluble residue is probably either arsenic or arsenic trioxide.
There's a really simple test for As in solution. It used to be used as a forensic screening test. To test the supernatant liquid for As, dip a piece
of thick gauge copper conductor wire in it. Allow to stand overnight. Copper reduces As compounds and the wire will turn black (plated As) if As is
present. It does this also with Sb for which I tried it and it works.
If the solution is very strongly acidic you may have to partly neutralise it for this test, or your copper will dissolve.
[Edited on 3-5-2014 by blogfast25]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
First, I added a bunch more skutterudite, which once again reacted (proving that there was still acid present). This gave another ruddy orange-brown
solution, indicating ferric nitrate. Once this had died down, I attempted to dilute and filter this out, obtaining a yellow, cloudy solution (iron
hydroxide precipitating from lower pH?). I added the copper wire whilst filtering, and more of this strange precipitate appeared. The color appears to
be anything from off-white to peach, due to the color of the solution. Going to do a control test with no copper wire to determine if that's the
cause...
On a side note, added the rest of my nitric acid to the leftover skutterudite particles, where it vigorously reacted (releasing lots of colorful fumes
of NO2). The same red-brown solution was obtained, and the insoluble residue on the bottom was dark gray in color. Some insoluble residue
appears to be floating, and white in color.
What an odd experiment!
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Is your mineral gangue free? Gangue could explain some things.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
There was nearly no gangue present, apart from a small green deposit on the surface.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The yellow cloudiness is almost certainly iron (III) hydroxy thingymejibs: not necessarily fully formed Fe(OH)3 but on its way there...
|
|
|
| Pages:
1
2
3
4
5
6 |