| Pages:
1
2
3
4
5
6
..
13 |
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Na doens't alloy with steel. OTOH if that little hole gets bunged up you have made a complicated pipe bomb.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
About the NaOH/Al method... Apparently sodium oxide's lattice energy is so low and Al2O3's is so high that the reduction of Na2O by Al is
exothermic! And combination of sodium oxide + oxygen to form sodium peroxide is exothermic too!
This is what I think happens:
1) Al reacts with NaOH:
2Al + 6NaOH => 2NaAlO2 + 2Na2O + 3H2
2) Then Al reacts with Na2O:
2Al + 3Na2O => 6Na + Al2O3
3) Finally, Al2O3 combines with Na2O:
Al2O3 + Na2O => 2NaAlO2
Overal:
Al + 2NaOH => Na + H2 + NaAlO2
Maybe aluminium does reduce sodium aluminates - my previous version.
|
|
|
guaguanco
Hazard to Others
  
Posts: 216
Registered: 26-11-2003
Member Is Offline
Mood: heterocyclic
|
|
| Quote: | Originally posted by chemoleo
If we ignore the formation of NaAlH4 for the moment,
[Edited on 8-12-2003 by chemoleo] |
That's safe to do, because I really,really doubt you've produced LiAlH4. LiAlH4 tends to be pyrophoric in air; I'm certain that hot
LiAlH4 would react instantly with O2, and possibly even with N2.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Experimentals on Sodium Thermites.
During holidays, I did two experiments on sodium thermites.
1. 6 g Al grains (filings, 0.1-0.5 mm) and NaOH prills (9 g) were mixed, in accordance with Al koholics post earlier. The experiment was pretty much
done as he did, a small iron soup can (roasted so that the label would burn away) whcih contained the mix and a tuna can on top that served as a lid.
This was heated with a strong propane gas flame, from below. After a minute or so, reaction ensued, gas came out between the two cans (lots) and
ignited instantaneously from the propane flame below. Colour was nicely yellow. At the same time, the bottom part of the can started to glow
red-orange, indicating that a reaction really was taking place. After 15 seconds or so, gas evolution stopped, and the red glow disappeared.
Being very pleased with the result, I allowed the can to cool in the snow. When I opened it later, it did NOT ignite or anything. Neither, to my
disappointment, did I see any sodium metalic globules on the side of the container  - in fact, everything was still in the bottom of can, a grey hard substance. This I removed from the can, and put a chunk into water. Wow, lots of gas
evolved, which sometimes ignited UNDER the water! Then I placed the rest into hot petroleum and boilded it at 110 deg C for 30 minutes (sodium melts
at 98 deg C or so). Sadly, nothing happened to it at all, no metal sodium floating out of the grey chunk
- in fact, everything was still in the bottom of can, a grey hard substance. This I removed from the can, and put a chunk into water. Wow, lots of gas
evolved, which sometimes ignited UNDER the water! Then I placed the rest into hot petroleum and boilded it at 110 deg C for 30 minutes (sodium melts
at 98 deg C or so). Sadly, nothing happened to it at all, no metal sodium floating out of the grey chunk  ... ...
so, what is this stuff really? I am thinking the Na may be so intimately mixed with the Al2O3 that it could not be melted out... alternatively, maybe
the excess of Al caused the formation of NaAlH4 rather than free Na.
To test this, I repeated the experiment, and used stoichiometric amounts of NaOH and Al. Same story again, the reaction product looked the same after
the reaction, and again did not liberate free Na after heating it in petroleum  ...
Oh, and then I took a small piece of the grey stuff and held it into a Bunsen flame. It didnt burn, not even glow really, so I am pretty sure there
wasnt much free Na or NaAlH4 around. Yet, the fact that it reacted so violently with water contradicts this.... I am confused ...
Oh, and then I took a small piece of the grey stuff and held it into a Bunsen flame. It didnt burn, not even glow really, so I am pretty sure there
wasnt much free Na or NaAlH4 around. Yet, the fact that it reacted so violently with water contradicts this.... I am confused 
2. The same story, but instead of Al I used 200 mesh magnesium (so no grains/filings like BromicAcid once did). As soon as I put the propane torch
onto the mix (from below, so the mix never contacted the open flame), it ignited, making a massive flash of light, and spewing molten white glowing
bits everywhere!! Whoa, I was lucky that I didnt get burned! Strange though that this was such a violent reaction! I struggle to believe it was only
due to the finer grade of Mg, as the NaOH prills have at least a 1 mm diameter anyway! I was thinking this is a great mix for easy flashpowder (ahem,
flashprills), doesnt need any oxidiser, just needs simple NaOH!
Needless to say, I didnt isolate any sodium from that experiment either....
Slightly discouraging, those two experiments, I know... I am yet waiting to see someone isolate appreciable amounts of Na that way!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Well chemoleo, I was waiting for someone to do the Mg-NaOH thing, since I don't have any Mg.
I really expected it to work after what Bromic acid did.
Shame.
I had the same problems with Al-NaOH, read my earlier post. As I said there, I don't think anyone will manage to make any Na that way, but with
Mg... Well, maybe someone can improve it.
Wish I had some Mg.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
chemoleo
You should have grinded your NaOH as fine as possible - carefully and preventing uptake of water from the air of course and mixed this with the Al/Mg
powder. Wrapping the can with rockwool might be favorable too.
A simple question of heat and heattransfer.
Thats my suggestion here.
[Edited on 24-1-2004 by Organikum]
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I have been thinking about the magnesium-NaOH. BromicAcid says it is described as a very energetic/explosive. Chemoleo, by experience, describes it
as a dangerous explosive flash. Well...
I gave up finding magnesium: disk drives structures are not made of it (I tested many kinds), I can’t find any scrap chainsaw structure, and the
soldering rods for car weels are simply not used anymore, nobody sells them here. Chemical supply is too expensive.
Seems I'm out...
If anybody wants to give it a try (carefull!), I would sugest two things: use Mg and NaOH in pellets, about 5mm diameter and think about using some
inert low-melting-point salt as a “dilluent” for the NaOH. My ignorant intuition keeps me thinking about anhydrous sodium silicate. What would be
its melting point?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
The diluent idea may be worth pursuing! I wish I had some Mg here right now, and I would try it!
I dont think grain size is an issue - as I used NaOH pellets (1mm diameter) anyhow - and yet it produced a very sensitive flash!
Tacho, have you checked ebay? They sometimes sell Mg filings, in bulk. Keep us up to date with your endeavours!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
| Quote: | Originally posted by Tacho
I have been thinking about the magnesium-NaOH. BromicAcid says it is described as a very energetic/explosive. Chemoleo, by experience, describes it
as a dangerous explosive flash. |
Does anyone have the equations for the reactions that are presumed to take place? If so, I could calculate the amount of heat liberated per gram. I
tend to think it is going to be less than most thermites due to the higher heats of formation of the oxides alkali metals than metals such as Fe/Cu.
Although if hydrogen is produced at some point in the reaction that could explode when mixed with air.
Hodges
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Just wanted to show the picture of the setup I used to do the thermite ignition/condensation. Its attached.
BTW- everything is made of stainless steel.
[Edited on 6-2-2004 by Tacho]

|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
The glass doesnt break?? 
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
BromicAcid
I just had time to read the text you posted and it's absolutely fantastic! I'm embarassed I haven't read it before. Anyone interested
in sodium making should read it, so I post the link again:
http://members.aol.com/bromicacid/sodium/index.htm
Chemoleo
E-bay is not an option. Delivery overseas would make it too expensive, or, at least,more expensive than a local chemical supply which sells reagent
grade stuff.
Saerenyde
I edited my post. No glass involved, just ss.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I think I made a sodium alloy using the themite.
I will be careful this time, no more false alarms.
After I read the wonderful BromicAcid`s refs, I decided to try to reduce NaCl+NaOH using Al. I mixed equal volumes of the salts and added Al powder
until “it looked right”. I did not use the big cup that shows in the picture of my previous post, but a smaller “cookie pan”.
Cool water in the dish... Blowtorch at the pan... Orange flames...Blowtorch off... Cool a bit and...
At the botton of the dish, on the condensation area, there was a gray deposit. Scratching this deposit , I noticed It was powdery, not metallic.
Anyway I gathered a decent amount of it ( 1g?) and slowly added to water. Orange sparks popped every time the dust grains touched the water. A bigger
chunk fizzled floating around the water in flames, the way I would expect sodium to do.
I repeated the experiment with the same results.
I would guess there is at least 30% elemental sodium there.
I’m trying to be cool, since I was wrong last time, but I am very excited.
I tried to reduce pure NaCl with Al, but there was no ignition, so I did not look for condensates. Maybe its a slow thing. We’ll see.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Hmm, but that's exactly what I got too!
A grey substance that would ignate on water contact! But I failed to isolate any pure Na - see my post above!
I think, much better may be to do the same thing with Mg, and add NaCl as a diluent to calm down the reaction. I wish I had some Mg here 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I have made sodium alright!
I mixed NaOH+NaCO3+NaCl, about a teaspoon of each, plus enough Al powder to make it “look righ”. The thermite was harder to start, but
once it started it was stronger than usual.
Beautiful chunks deposited in the condensation plate. Scratched to a 50ml centrifuge tube with about 20ml of xylene. This time it felt like a soft
metal being scratched. Metallic bits everywhere. About 2ml of powder.
I am disappointed because it did not melt into one blob under heat. It remained a powder but, oh boy, pick a chunk of that and throw in water!! BIG
FAT FLAMES!
If that is not metallic sodium I don’t care. Is good enough for me.
Lye, common salt, pool’s carbonate.
10 minutes, little smoke, indoors, simple reactants.
Believe me chemoleo: this is different! it's not THAT grey thing!
I believe the NaCl and NaCO3 are being reduced by the Al, like BromicAcid's papers mention, the NaOH-Al thermite just gives the heat and
produces the gray garbage you mention. THIS IS NOT THE SAME THING!
Try it!
[Edited on 7-2-2004 by Tacho]
[Edited on 7-2-2004 by Tacho]
[Edited on 7-2-2004 by Tacho]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
How bout leaching out the sodium with mercury and distilling of the mercury under reduced pressure?
Hazardous and toxic, I know, but hey, fun for sure!
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
WOW, good job  Now I really want to try this sometime. I just need to find
aluminum powder Now I really want to try this sometime. I just need to find
aluminum powder 
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
Might you have developed any weight ratios as of yet? Add Al until it looks right can be a little hard to reproduce.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Mumbles,
You are right, but I can't offer you proper measures yet. I will post a decent description as soon as possible.
If anyone wants to try, I estimate the Al powder volume in about 2/3 the volume of the salts (1vol NaOH, 1vol NaCl, 1vol NaCO3, 2 vol Al powder). That
proportion will surely give you results to work with.
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
| Quote: | Originally posted by Tacho
I mixed NaOH+NaCO3+NaCl, about a teaspoon of each, plus enough Al powder to make it “look righ”. The thermite was harder to start, but
once it started it was stronger than usual.
Beautiful chunks deposited in the condensation plate. |
This sounds interesting. How did you ignite the thermite and then get the condensation plate over it in time?
Hodges
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
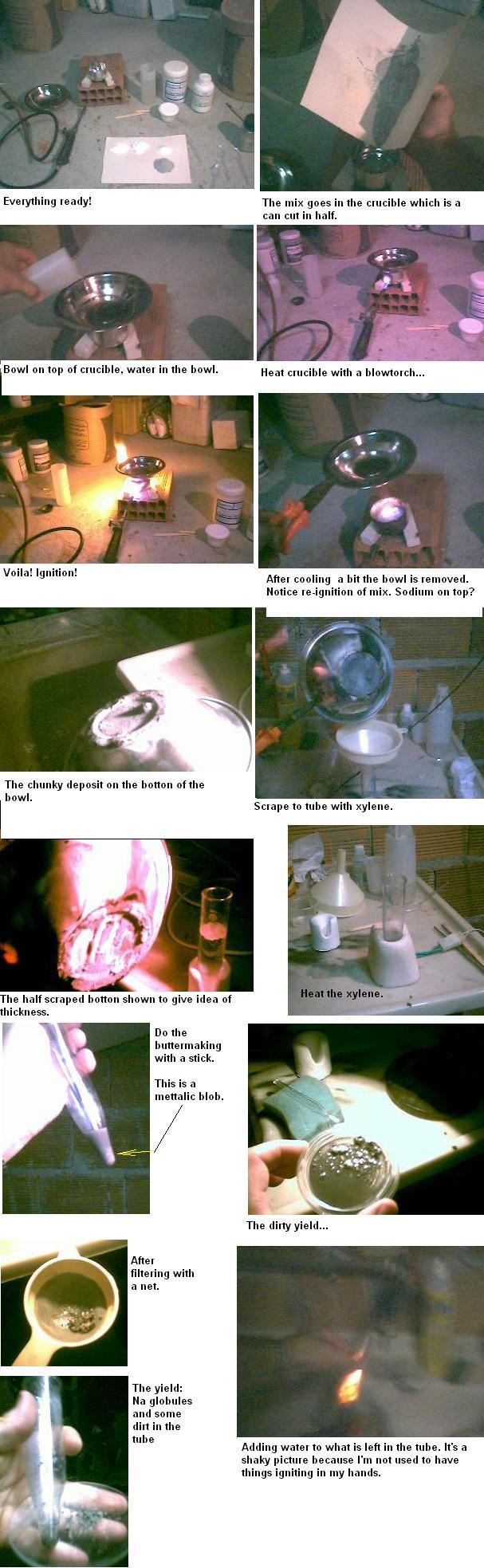
Obtaining metallic sodium nuggets using sodium salts and powdered aluminum.
Mix 20g powdered aluminum, 6g NaOH, 3g NaCO3, and 5g NaCl in a steel crucible.
Put the crucible over a heat resistant base that allows heating by flame. Cover the crucible with a steel bowl filled with water (check attached
picture, but don’t do it over you computer).
Heat the crucible vigorously with a blowtorch or a heavy duty bunsen burner until a reaction takes place, marked by yellow flames escaping from the
crucible.
When the reaction is over and the crucible is no longer red, remove the bowl carefully pouring the water away. There should be a light grey deposit at
the botton. Scrape this deposit with a spatula to a 50ml vial with some xylene in it using a funnel. Repeat this procedure four times.
A warning here: you now have metallic sodium everywhere! Be careful. When you wash the crucible, the bowl or the funnel, orange flames and loud pops
might show. Specially the residues in the crucible: there is a lot of sodium left there. You have to wash and dry them because the residues absorb
humidity from air very fast.
Heat the xylene to ebulition and then turn off the heat. While the xylene cools, using a glass rod (I used disposable wood chopstick) keep revolving
the sediment, doing compressions as well as stirring. Slowly. I can’t help thinking of butter making. You should end up with half a dozen
metallic nuggets and a gray residue.
My yield was 0,3g for five runs. Enough to cover a fingernail with nuggets.
Notes:
1- The thermite reaction is harder to start than the pure NaOH-Al.
2- The little sodium nuggets are very soft, as expected, and don’t produce flames when tossed in water, they just run around quickly on the
surface, fizzing until they disappear with a pop and a spark. Careful with the residue in the crucible though, not only it can ignite again when you
remove the bowl, but it can promote some fire when washed.
3- I don’t know the mesh grade of my Al powder, but it looks like grey coarse alumina, or very fine sand. I bough it in a fiberglass supply
shop. It is supposed to get mixed with polyester resin for some purpose. It is not that superfine kind that would stick to a glass like paint. It
falls off a glass surface leaving just some dirt behind. My NaOH comes in 1-2mm pellets.
4- I tried twice to melt the sodium without the xylene. First time I got a little nugget for 1 run but ruined the glass tube. Second time the sodium
ignited and I ruined the glass tube.
5- Everything is extremely empirical. The weights of the reactants are basically based in the volumes seemed to make better results, that is: 1 vol of
NaOH, 1 vol NaCl, 1 vol NaCO3 and 2,3 vol Al.
This is all I learned in the last 24 hours. A total of 3.456 improvements can be made to the process.
BTW- Please take a look at my “Versatile miniscale glassware” thread in the reagent aquisition and apparatus forum. I don’t think
it’s getting the views it deserves. I wish I had seen something like that when I started my hobby.

|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Pictures of the whole thing.
Voguel’s third edition says that the way to make sodium POWDER is to heat it in xylene and stir. No wonder I can’t get the sodium to melt together
in a big blob. This is the main problem I find. I bet more than half that powder is sodium, just have to find a way to melt it in one piece. Any help
would be appreciated.
Here goes the full thing in pictures:
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Do think i had had a potential termite mixture at home.. My drain cleaner has
NaOH and Al pieces together in the same bottle. All ihave to do is to pour into a soup can and heat... My drain cleaner has
NaOH and Al pieces together in the same bottle. All ihave to do is to pour into a soup can and heat...
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
To obtain sodium granules you must mix NaCl to the NaOH. Some NaCO3 seems to improve yields. See my post some time ago.
Anyway, I always used aluminum POWDER. I would be surprised if those chunks of aluminium make a violent reaction. If you really want to try, maybe
shreded thin Al foil. I Never tried it.
Be very careful, many things can go wrong!
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I went out experimenting today and here are the results. I tried chemical reduction of NaOH with Aluminum but the shavings were too big. I was
hoping that extensive heating would liquify them but after 15 minutes with the torch I gave up. Oh well. After that I hooked up my power source to
try some electrolysis. I know that I've read of only a few solvents that can be used to produce sodium but I decided to do some tests of my own.
I tried NaOH in acetonitrile, kerosine, mineral oil, and toluene with no results, wasn't expecting any anyways.
Finally though I tried electrolysis of NaNO3 like I mentioned in the patent a few posts back. So I used the same procedure as before, put electrodes
in NaNO3, spritz with water to make conductive, pump up the voltage. Immediately the NaNO3 started turning green, then the water started to evaporate
off from the heat and NO2 started coming up. I grabbed a strip of copper and held it above the test tube out of curiosity and sure enough it started
eating it, good ol' nitric acid. So I ran it a bit longer and it was really really eating into one of my electrodes. Nickel really isn't
resillant to oxidation so it was dying a horrible death.
I let it run a bit more but was getting nervous as it started to melt and work all of the water our of it's system. Regardless of the patent I
still have a slight phobia that the Na/NaNO3 mixture that would result would explode like flash so before it totally dried out I discontinued
electrolysis.
This weekend I am going to do some major sodium research, I've got two methods that I want to try for mass production. Everyone cross your
fingers! 
[Edited on 4/29/2004 by BromicAcid]
|
|
|
| Pages:
1
2
3
4
5
6
..
13 |