| Pages:
1
2
3
4
5
6 |
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
He MELTED the fuse in his hotplate
No suckback trap
Mercury crud everywhere
He even has a Darwin logo on his website *facepalm* Darwin Award!
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Isn't it bad to have teflon at the joints? I've heard teflon expand quicker than borosilicate and could cause a break.
Also, I'm quite shocked at the amount of mercury those guys have. They say they are doing a job for someone so i guess a company gave it to them so
that they would distille it and return it back.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
It's teflon tape, it's ok. It's pretty much the only right thing they did.
I doubt it was a company. Probably someone's private batch. A company would've done it professionally. There's simply too much of it.
I can literally imagine my anxiety while carrying that large reagent bottle, waiting for the bottom to fall off and bathe me like Terminator. Man,
they're out of their minds.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I have bought another 100g of finely powdered cinnabar and I will make more mercury using different process such as air roasting, calcium carbonate
reaction etc. Yield will be calculated and Air roasting process will be documented in my short book that should be online soon named:
Lab scale industrial process
For lab synthesis 2012
Plante1999
I will keep you informed!
I never asked for this.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
I would love to be able to distil mercury. However, my equipment is a bit rudimentary and it may be dangerous.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yesterday i boilled some cinnabar and conc nitric acide (just to see if i could ..)
and some water.
i got a little bit of fizzing ( thinking it was H<sub>2</sub>S bubbling up) but then after cooling i obtained this yellowish oily residue
and a faint smell of sulfur
(not rotten eggs !)
but still not reaction from the cinnabar...
i thought maybe...HgS + 2 HNO<sub>3</sub> = H<sub>2</sub>S(g) + Hg(NO<sub>3</sub> <sub>2</sub> <sub>2</sub>
since one of the product leaves the reaction shouldnt the reaction take place?
any help/thoughts?
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: subscript;
rxn. eqn. cleanup]
[Edited on 3.8.13 by bfesser]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Generally, when sulphides are dissolved in nitric acid, it yield a nitrate salt and sulphur. You would be better to extract liquid mercury from the
cinnabar, purify it, and then react it with the nitric acid.
I never asked for this.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
well yes that was exactly what i was aiming for! but it didnt happen!
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I guess the yellow thing is sulphur, no? What is your desired product, and how much cinnabar do you have?
I never asked for this.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
if it is indeed sulphur then the reaction is painfully slow even at boilling point and needs constant re addition of acid/water..
i have about 50 g of cinnabar only about 1-2 grams were involved in this .
how did i arrived at S8?
my goal is to produce Hg with wet chemistry i dont want to roast the thing ....(too many kids live down wind)
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
as previously mentioned, i was looking for a way to isolate mercury without roasting .
a wet chemical way was suggested elegantly by Plante1999.
72g of NaOH were added to 9.6 g of finely powdered sulfur and 200 ml of boiling water.

the solution remained yellow for a while and then turned deep orang/redish

after cooling 48.8g of cinnabar were added
strong stiring is apply when adding aluminum powder (or foil)
not too much just a little at the time ...or this will happen

when no more cinnabar is visible, i obtained a slushy metallic goo of aluminum and mercury alloy

a dilute solution of KMnO<sub>4</sub> brings the bright color of metal back
after several cleaning [HNO<sub>3</sub>] is added cleaned and HCl finish the job.

when no more bubbling is apparent, i had 37g of clean mercury metal.
i arrived at a 75.5% of what i put in which is pretty good for a sloppy chemist like me!

this reaction was thought of by Plante1999 and sudgested as a mean to obtain mercury from cinnabar through a wet process
this reaction is very eficient and deserve attention although not practical on an industrial scale because of the amount of waste chemicals being
produce but on a lab scale is fast cheap and generate good purity mercury.
thanks to plante1999 for the idea
oh and merry chrismas to all...
[Edited on 25-12-2012 by neptunium]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: subscript;
"HN03"; whitespace]
[Edited on 3.8.13 by bfesser]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i must add that the mercury had to be THROUGHLY rinsed with water to eliminate all remaining traces of acid
otherwise it will form crystals and loose its shine..
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
ran the experiment again and combined the total Hg...
over 80g of mercury metal! and pretty shiny and pure!

|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
That's a very interesting method neptunium. Good to see that there is also a viable wet chemical method to prepare mercury from HgS.
What you're doing is forming a solution with thiomercurate anions and then reducing this with aluminium.
The purity of the mercury thus obtained should be checked, as many of the possible alloying elements in aluminium foil could stay with the mercury.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Well, I thought/ tried this process few month ago based on something pretty vague I read somewhere. When I saw that neptunium was in need of an wet
method, because he didn't wanted to make mercury vapor. I told him my process in an U2U and some emails. I didn't posted it because it lacked of
pictures, and some may have said that it was non working because they could not see picture of it.
There is not only the presence of element in the aluminium foil, but also in the ore itself, that generally contain gold, lead and arsenic. However
the purifying process I recommended (which avoid distillation) should remove most metal which are less noble than mercury, leaving mostly the Ag and
the Au in it. No test was done for the purity tough. (At least in my case).
I never asked for this.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Where do you buy powdered cinnabar?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
On ebay there is a china supplier. Is name is Gaofudev.
I had very good service with him, but he is quite costly.
[Edited on 31-12-2012 by plante1999]
I never asked for this.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes thats the one i had the cinnabar from...i purified the mercury alloy with a dilute solution of KMnO<sub>4</sub> and then HCl conc.
it bubbles for as long as Al is present (about 30 to 45 min) and then rinse it with water and strong agitation for about 1 hour..
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>:
subscript]
[Edited on 3.8.13 by bfesser]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
You should check the composition of foil before saying this purification treatment will fix it. I think the treatment has to be done right (lots of
shaking) in order to remove most contaminants (I've posted this), but it might not work here in any case.
Brauer recommends glass for distilling mercury (interesting method), and so do the Biltzes: Heat a mixture of 23 g. cinnabar and somewhat more
than the calculated amount of iron filings in a small retort of difficultly fusible glass. The mercury distils into a small flask which serves as
receiver. Yield, 16 to 18 g.
I keep my mercury in glass and used to have a big glass flask of it...just saying I didn't have a problem with the video, except they needed a shorter
path and better insulation...if they didn't use water in the condenser someone would probably say they should have...though I wonder why it ended so
soon...and whether they know how toxic mercury vapor is.
[Edited on 31-12-2012 by S.C. Wack]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Plante1999 uses foil i used pure very fine aluminum powder 500mesh. maybe i forgot to clarify that detail
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
In fact at the beginning I used foil, but the reaction was too violent, so I used 1/8 Al sheet pieces later on.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quote(s)]
[Edited on 2.8.13 by bfesser]
I never asked for this.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by plante1999  | Edit: I will probably try this reaction:
4 CaCO<sub>3</sub> + 4 HgS --> 4 CO<sub>2</sub> + 3 CaS + CaSO<sub>4</sub> + 4Hg
Calcium carbonate is easier to get than Iron powder...
This is a varient of a process that I saw in many documents. |
The following must be equal:
4 CaO + 4 HgS --> 3 CaS + CaSO<sub>4</sub> + 4 Hg
4 Ca(OH)<sub>2</sub> + 4 HgS --> 3 CaS + CaSO<sub>4</sub> + 4 H<sub>2</sub>O + 4 Hg
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: rxn. eqn.
cleanup
[Edited on 3.8.13 by bfesser]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
bfesser
|
Thread Topped
2-8-2013 at 11:54 |
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Mercury Purification [lit.]
Quote: Originally posted by blogfast25  | | You can purify mercury to some extent by keeping it under dilute nitric acid, I seem to recall a lab procedure consisting of dripping contaminated Hg
through a column of SG = 1.1 HNO<sub>3</sub>. The contaminant metals mostly dissolve in the nitric, leaving behind purified mercury.
|
I was a little bored, so I transcribed a few pages from a book in my personal library: | Quote: | <em><strong>Appendix 8</strong></em>
<div align="right"><em><strong>Purification and Decontamination of Mercury</strong></em></div>
Dirty mercury contains
dissolved metals, as well as organic materials, grease, oxides, and just plain dirt, which are usually found on top and in pockets trapped under the
surface.
The three steps in the purification process are: filtration, to remove grease, dirt, and
some dissolved metal; oxidation, to remove base metals; and distillation, to remove noble metals.
First, pass the mercury through a pinhole in a cone or funnel of filter paper or through
glass wool to remove dirt and some dissolved metal. The heavy mercury falls through the pinhole, while dirt, oxide, and grease will stick to the
paper and remain behind. As the mercury falls through the pinhole the surface layer in which metallic impurities are concentrated will contract. The
impurities will concentrate further in the contracting surface and lower the surface tension so that the impure surface mercury does not pass through
the pinhole. Each passage of mercury through a pinhole is equivalent to partial extraction. A dozen or so repeated filtrations will purify mercury
sufficiently for most purposes. However, it is usually better to filter once or twice and then to remove most active metals by oxidation.
<table><tr><td> This is done by forcing air through the mercury. The
simplest apparatus is shown in Diagram I. Put a layer of 10–20% nitric acid over the dirty mercury and pull air through by an aspirator or
vacuum line. The air will oxidize the base metal such as zinc, lead, etc., while stirring the mercury. The oxides will float to the top of the
mercury and dissolve in the acid solution. The nitric acid will also oxidize any base metal at the mercury surface. The bubble rate should be fast
enough to stir the mercury without splashing it into the side arm. The glass wool plug is present to protect the aspirator or vacuum line from acid
droplets. Usually 8 hours of oxidation at the rate of two to three air bubbles per second will clean about 10 pounds of mercury.
After the oxidation wash the mercury with distilled water. Then pass it through a pinhole
in a filter paper to remove the last traces of oxides. For most purposes the mercury will now be sufficiently clean. For example, the gold,
platinum, or silver still present will not affect routine density measurements or interfere with use in mercury contacts or seals.
When very pure mercury is require, as in a polarography and in surface tension
measurements, after the oxidation vacuum distill the mercury at 25–30 mm pressure. Use an all-Pyrex apparatus with a glass shield and
distill in a hood in case the apparatus breaks.</td><td>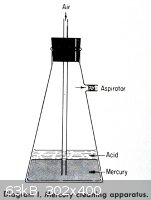 </td></tr></table> </td></tr></table>
<strong>REMOVAL OF SPILLED MERCURY</strong>
Spilled mercury on desk tops and in cracks in the floor is an <em>extremely
dangerous health hazard.</em> Mercury vapor is poisonous, and if inhales attacks the nails, hair, teeth, brain, and kidneys. To avoid
spillage, work with mercury over wide-mouth safety jars or pots. Place apparatus on trays so that if a container breaks and the mercury spills it
will be caught. To remove or deactivate spilled mercury, use sequestering agents, chemicals which lower the vapor pressure of the mercury and convert
it into a form that can be swept up. (Mercury splashes into droplets to small to be seen and too fluid to be swept up.)
Cover the contaminated areas with either sulfur or some other sequestering agent, such as
an organic sulfur compound. Sulfur, over an extended period of time, cuts down the vapor pressure by combining with the mercury to form solid mercury
sulfide, which may be swept up. Organic sequestering agents do not contain free sulfur but hydrolyze to form H<sub>2</sub>S, which then
reacts with the mercury. Dissolve the sequestering agent in water and wash the floors and desks with the solution. Vacate the room for a period of
time to avoid the hydrogen sulfide, which is an even more deadly poison than the mercury. After the room is ventilated, sweep up the mercury sulfide.
– <em>Laboratory Course in Physical Chemistry.</em> Salzberg, Morrow, & Cohen. Second Printing, Academic Press, 1966.
(<a href="http://books.google.com/books/about/Laboratory_course_in_physical_chemistry.html?id=jsc0AAAAMAAJ" target="_blank">Google
Books</a> <img src="../scipics/_ext.png" /> |
[Edited on 3.8.13 by bfesser]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Thanks bfesser for the handy references. It will help keep member safe.
Very glad the thread is toped.
By the way, I didn't updated much this thread, but I did proceed a lot of cinnabar since my last reported try.
The process I invented/tried:
-Wet sulphur increased solubility aluminium reduction. (Personally developed)
It was very messy, and pretty violent, but the yield is good and no mercury vapors are involved. Mercury is of OK purity. Costly.
- Sodium/calcium carbonate, and heating, at low temperature the yield is very high and the mercury is of high purity, but as temp increase, the
carbonate/sulphur mixture decompose back to sulphur which spoil the mercury. By far the best process if a careful chemist do it.
- Iron filling, give Good mercury, pretty easilly but is a mess to clean, very good for a sloppy chemist.
- Air oxidation (Look in lab scale industrial process 1). By far the cheapest and nice(st) to look at, mercury fog rising from the red cinnabar and
driping back is simply the most beautiful thing I saw in my whole life honestly. Deal with heavy apparatus and mercury vapors.
-Aluminium/Zinc
EXTREMELY dangerous, explode making a quite loud sound, with moderating agent or filling burn extremely fast like cellulose hexanitrate making a dark
coating of suspected mercury sulphide due to hydrolysis of the poor metal sulphide. it is beleived that the mercury is trapped in the sulphide(s).
-Wet process using aqua regia. Extremely costly. Dissolve cinnabar in aqua regia, filter and precipitate with copper. Gives very good purity Hg but
mess with strong mercury solution. Best process for the riches out there.
I never asked for this.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Hi plante. I see this a quick summary of some of the methods discussed in the thread and you have implemented yourself,
I wonder of you would mind putting a few hard figures on your yields. Further how did you characterise purity?
Surely you would not state 'high purity' just because product looks nice and shiny?
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
| Pages:
1
2
3
4
5
6 |