| Pages:
1
2
3
4 |
Neil
National Hazard
   
Posts: 556
Registered: 19-3-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ozone
Any concentration (1%, even) IV will be instantly broken down by catalase and iron compounds in the blood. This will yield O2 (g) which will (likely)
yield clots and death via embolism. Bubbles in the blood stream are bad, mkay!
Greater concentrations should only add to the spectacle, not the final result . .
WTF?
O3 |
Google it... 'oxygen revitalises and refreshes you' or some bs like that... people have been there done that...see here...Scary eh?
Edit: as a side note to Canada's moderation of 35% H2O2, buy it from hydro stores with cash.. they don't keep records of their purchasers for some
reason ;o)
[Edited on 9-4-2008 by Neil]
|
|
|
pbmineral
Harmless

Posts: 34
Registered: 25-5-2007
Member Is Offline
Mood: No Mood
|
|
Hi everyone,
I had seen in the Handbook that pure H2O2 could be made by extraction of commercial solutions using ethyl ether thjat extracts only pure H2O2, never
tried it !
Any suggestions ?
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
An interesting patent, pitty its jap. It seems its just an acid stabalised H2O2, though it would be interesting to see what concentrations are
optained. The reference to it exploding suggests high concentrations.
<b>Concentration of hydrogen peroxide water by heating.</b> Taketa, Kikuo; Watanabe, Satoshi; Sasaki, Shigeru; Naka, Hideo; Okusaki,
Junichi. (Sumitomo Chemical Co., Ltd., Japan; Sumika Bunseki Center K. K.). Jpn. Kokai Tokkyo Koho (1998), 5 pp. CODEN: JKXXAF JP 10338508
A 19981222 Heisei. Patent written in Japanese. Application: JP 97-145447 19970603. Priority: . CAN 130:68703 AN 1998:816487 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
JP 10338508 A 19981222 JP 1997-145447 19970603
Priority Application
JP 1997-145447 19970603
Abstract
HCl is added to aq. H2O2, and then the soln. is heated for concn. The HCl addn. prevents explosion of the aq. soln. during the concn.
[Edited on 27-5-2008 by Axt]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by pbmineral
Hi everyone,
I had seen in the Handbook that pure H2O2 could be made by extraction of commercial solutions using ethyl ether thjat extracts only pure H2O2, never
tried it !
Any suggestions ? |
I've done some of these with various concentrations but it was a wasn't worth the ether IMO. First you should know that the mixture is detonable, but
even more hazardous is the potential of formation of risky organic peroxides and lastly you have to use a very large amount of ether to extract any
significant amount
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
| Quote: |
Google it... 'oxygen revitalises and refreshes you' or some bs like that... people have been there done that...see here...Scary eh?
|
Read up on the details of this story. IMO, it was virtually impossible for her death to have been caused by that little H2O2. 25mL of O2 killed her
4 days later? Bullshit.
I've been thinking about the barium peroxide method for producing H2O2. How is barium peroxide obtained?
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MagicJigPipe
...
I've been thinking about the barium peroxide method for producing H2O2. How is barium peroxide obtained? |
Basically by heating barium oxide in CO2 (and SOx NOx) free air. Usually done under a couple of atmospheres of air pressure at 500 to 600 C. An old
process for extracting oxygen from the air started with this, then while keeping the temperature the same pumping off the air and then the released
O2; repeat until BaO becomes too unreactive.
The BaO is made by heating a mixture of BaCO3 and carbon, made into a paste with oil or tar, to yellow to white heat. The plain carbonate does not
decompose readily until much higher temperatures, the carbon causes a decomposition to carbon monoxide and barium oxide at a lower temperature.
lets see now, here's one old reference, there are plenty of others from this time and earlier.
http://books.google.com/books?id=MAA5AAAAIAAJ&pg=PA640&a...
|
|
|
VestriDeus
Harmless

Posts: 16
Registered: 15-11-2009
Member Is Offline
Mood: No Mood
|
|
Has anyone tried this:
http://www.instructables.com/id/Distill-Hydrogen-Peroxide/
It says to add iodine-free table salt to low conc. H2O2, and that the stuff should separate into two layers, salt water and hydrogen peroxide.
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
I dont want to reject the possibility directly, anyway that guy that wrote the stuff does not really know what he's talking about.
"Distill Hydrogen Peroxide"
Distilling?, what is he distilling?
It could be that NaCl is fully insoluble in hydrogenperoxide, in this case it could be possible to force the H2O2 out by saturating the H2O.
Can anyone support this ?
What a fine day for chemistry this is.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Way to contaminate with metallic cations. Sodium chloride is a catalyst for H2O2 decomposition, not as strong as Pt, Ag, Os, MnO2, etc. but in the
same order as CuO, Mn(NO3)2, HgO, AgO, FeO, ZnSO4, Na2CO3, CaCl2, BaCl2, etc. Nothing there even hints that a more conc. peroxide results. It looks
like a joke.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
This more then likely has been suggested already but the idea has been floating in my head for about a week and with all this talk of H2O2
concentration going around how would this work out?
Placing an open bottle of H2O2 into a dessicator bag full of MgSO4 and sealed up in a dark place. It will eliminate the worry of organic dust falling
in while still allowing it to evaporate the water from the solution.
I suspect near 100% H2O2 could in theory be formed this way but perhaps some form of equilibrium will setup with water absorbant properties of H2O2
that I do not know about.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
I can see your point.
Often i wonder why such hard ways of doing stuff is considered.
Dessicating substances is such an old and plain simple way.
The same thing can be done with HNO3 if the absorbing properties are high enough.
[Edited on 17-11-2009 by User]
What a fine day for chemistry this is.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Sedit  | This more then likely has been suggested already but the idea has been floating in my head for about a week and with all this talk of H2O2
concentration going around how would this work out?
Placing an open bottle of H2O2 into a dessicator bag full of MgSO4 and sealed up in a dark place. It will eliminate the worry of organic dust falling
in while still allowing it to evaporate the water from the solution. |
The threat of organics (like cellulosics), is often due to present trace metal catalysts. So, I wouldn't dry H2O2 with metallic salts like MgSO4
because then of airborne particulates that would end up contaminating it. Sterile environment is crucial for high strength peroxide. P2O5 or H2SO4 are
good choices. Regular dessication works for higher strength, but takes a long time. Vacuum dessication is said to work even faster. There are other
conc. threads that talk about this in detail. I also suggest to see what Mellor has to say about concentration.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Your right Formatik although it can be solved with a little glass wool in the opening though. I hate the idea of drying stuff with H2SO4 for some
reason. I will look into Mellor and see what kind of data he gives although its not to important to me since I can get 35% with ease and thats more
then enough for most things for me. Very few synthesis call for 100% H2O2 so the rest is just bulk.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
I was going to concentrate some hydrogen peroxide by slow evaporation, but I noticed that the permissable exposure limit for the vapor is 1ppm,
according to wikipedia. I don't want to try evaporating it outside, because there is rain, dust, animals, wind, and so many other factors which make
it hard to leave an open tray of reagent. I already tried putting some in a freezer, but the liquid fraction was absorbed and 'lost' in a mass of
water ice (imagine a spoon of water in a cup of sand). If you evaporate a solution of peroxide inside, is the vapor really something to worry about?
I am surprised that it's considered safe to dab disinfectant with 3% liquid hydrogen peroxide on an open cut, but that a milliliter of that same
solution (1mmol H2O2) evaporated in a room with a volume of 20,000 liters is considered unsafe.
And no, I don't have a fume hood or vacuum distillation equipment, so those options are out of the question for now.
Put that in your pipe and smoke it!
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
What concentration do you need?
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by C6(NO2)5CH2CH(CH3)N(NO2)2  | I was going to concentrate some hydrogen peroxide by slow evaporation, but I noticed that the permissable exposure limit for the vapor is 1ppm,
according to wikipedia. I don't want to try evaporating it outside, because there is rain, dust, animals, wind, and so many other factors which make
it hard to leave an open tray of reagent. I already tried putting some in a freezer, but the liquid fraction was absorbed and 'lost' in a mass of
water ice (imagine a spoon of water in a cup of sand). If you evaporate a solution of peroxide inside, is the vapor really something to worry about?
I am surprised that it's considered safe to dab disinfectant with 3% liquid hydrogen peroxide on an open cut, but that a milliliter of that same
solution (1mmol H2O2) evaporated in a room with a volume of 20,000 liters is considered unsafe.
And no, I don't have a fume hood or vacuum distillation equipment, so those options are out of the question for now. |
Perhaps another hobby.
Can you point to death by too much hydrogen peroxide in the air?
Not on my worry list.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Did you try to filter this mass while still in the freezer perhaps?
You may be able collect a useable amount of product by letting it sit filtering overnight.
I mostly agree with morganbw on this one though.
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
Morganbw, it's not that I'm so worried about chemical exposure. I would like to think I could safely handle any vapor while inhaling less than 1 ppm.
But to do that, I'd use a combination of extra safety equipment (respirator cartridges?), good ventilation, and precautions to limit how much vapor
was emitted (e.g. I'd put it in a jar, in a sealed bag of dehydrated epsom salt or silica gel outside). But, if a material is not dangerous to inhale,
then I'm not going to waste my time money and equipment trying to keep the vapors out of my house. What led me to look up those exposure limits was an
idea I had to concentrate hydrogen peroxide conveniently. I thought about pouring it onto a plate in a parked car during summer, it will heat up to
60C more or less and some of the water would evaporate, and then I would pour the (now concentrated) H2O2 in a bottle and roll down the windows to get
rid of the humidity while driving home. The only question was I was unsure if it was okay to breathe air that had that much hydrogen peroxide in it.
Would it be okay to get in a car with 1000 ppm H2O2? would it poison me? So, I looked it up online: the answers are NO!, and maybe.
I'm still not sure why OSHA set the limit so low. For carbon tetrachloride and hydrogen cyanide the set it at 10 ppm, for nitrogen dioxide 5ppm, for
nitric acid 2 ppm, for ethylene oxide and elemental chlorine 1ppm, for phosphine 0.3ppm, and for chlorine dioxide .1 ppm. Bear in mind that these
values are all for all-day, occupational type exposure, if you have something with low acute toxicity like carbon tetrachloride you can have a bit
more during a short term experiment. But hydrogen peroxide seems like a safe, mundane chemical compared to all of those, so I don't know why they are
so worried about it.
I'm trying to concentrate a 3% hydrogen peroxide disinfectant, to a concentration of preferably over 15%, although 30 would be ideal. I don't need
rocket fuel here. Last night, I filled a 118ml plastic bottle half full with 3% solution and let it freeze on its side with the capped end angled
slightly up. It froze into a solid piece, and in the morning I turned it cap down and squeezed it to make some cracks in the ice. While I did that,
several milliliters of liquid ran out of the ice and gathered in the cap. The temp was -6C, and that fluid resisted freezing, so I think it is at
least 10% according to https://en.wikipedia.org/wiki/Hydrogen_peroxide#/media/File hase_diagram_hydrogen_peroxide_water.svg hase_diagram_hydrogen_peroxide_water.svg
The bottle is still draining , and the temperature is falling again, so I will see what concentration and amount is yielded. To check concentration I
will try to react a bit with MnO2 or yeast or something and see how much oxygen I get. I am kind of optimistic this time: the key I think is to not
freeze it to as low of a temperature (although you will get lower concentration that way) and more importantly, freeze it slowly so you don't get as
small of crystals to act as a sponge. If this fails I may try to filter out the ice as it freezes-that should get a good yield, and failing that, I
will find a way to evaporate it. I won't quite treat it like liquid chlorine, but I won't be setting a plate of it in my bedroom, either
Put that in your pipe and smoke it!
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
The liquid fraction was about 15ml, which tested at 30 volumes of oxygen using yeast, or around 10%. I should have got something like 18ml of 10% from
60ml of 3%, so about 3ml, or one ml per 15ml of water ice, was retained. That could make it hard for higher concentrations, since for 30% you will
only have about 1ml of product per 10ml ice. That means the majority of it could be absorbed in the ice. Next thing to try will be crushing it better,
and maybe exposing it to centrifugal force briefly.
Put that in your pipe and smoke it!
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Evaporation is OK.
Years ago, someone here posted a table.
H2O2 concentration may be easily determined by specific gravity.
Elaborate chemical tests aren't required. Just use a hydrometer.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Are there small hydrometer/hydrometer jars? (Just curious because right now I'm too lazy and watching a hockey game to get up and look in a catalog.)
If you have a balance that will weigh to 2 decimal places, and a 10.0 mL pipette or volumetric flask, you can measure density to 3 significant
figures, which I would think would give a good approximation of the density. How does the yeast method work?
|
|
|
wg48temp9
National Hazard
   
Posts: 783
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by C6(NO2)5CH2CH(CH3)N(NO2)2  |
I filled a 118ml plastic bottle half full with 3% solution and let it freeze on its side with the capped end angled slightly up. It froze into a solid
piece, and in the morning I turned it cap down and squeezed it to make some cracks in the ice. While I did that, several milliliters of liquid ran out
of the ice and gathered in the cap. The temp was -6C, and that fluid resisted freezing, so I think it is at least 10% according to https://en.wikipedia.org/wiki/Hydrogen_peroxide#/media/File hase_diagram_hydrogen_peroxide_water.svg hase_diagram_hydrogen_peroxide_water.svg |
If I have the above correct all I have to do is freeze my H2O2 water solution to form a mixture of solid and liquid, assuming the original strength of
the H2O2 solution is less than about 50% and the mixture has a achieved equilibrium , the liquid is a higher strength than the strength of the
original solution.
So for example if I freeze the dilute H2O2 solution in a freeze to say -25C (thermostat turned full on) then the liquid remaining will have strength
of 30%. Obviously I don't know how much H2O2 will be trapped in the solid but even if its 50% of the total H2O2 its not destroyed so it sounds like a
very practical method to obtain 30% H2O2, assuming you can get the freezer down to -25C. I will have to give it a try with my 12% solution preferably
with stirring to form a slush I can filter off the liquid easily.
Below is a more detailed phase diagram. It confirms that the solid is water ice above about -50C. Its also interesting that the maximum strength
achievable with this method is about 60% and that H2O2 forms an adduct /crystal structure between the H2O2 and water.
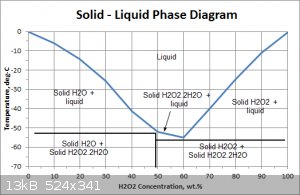
Sorry I have lost the reference to the file
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
| Pages:
1
2
3
4 |