| Pages:
1
2
3
4 |
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
TiCl3 is a unique reducing agent in organic chemistry; it tends to reduce nucleophilic substrates, while most reducing agents react with
electrophiles. So for example it reduces only aromatic carbonyls:
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/1099-0690(200210)2002:19%3C3326::AID-EJOC3326%3E3.0.CO;2-V
It also catalyzes a number of transformations of oximes, in particular allowing for a unique synthesis of tetrazole derivatives from oximes and sodium
azide:
https://www.sciencedirect.com/science/article/pii/S004040391...
Most of the interesting chemistry of TiCl3 requires the anhydrous compound and takes place in nonaqueous solvents. Luckily, I think this is one of the
metal halides that can be dehydrated by heating.
[Edited on 18-4-2020 by clearly_not_atara]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Very interesting experiment. TiCl3 indeed is strongly reducing. I wonder how pure it is. Probably it will contain quite some titanium(IV) as well. I
know from experience that a solution of TiCl3 in dilute HCl, when left in contact with air, slowly becomes colorless, all of the titanium(III) being
converted to titanium(IV).
The deep red material is a peroxo complex of titanium(IV). Addition of H2O2 first oxidizes the titanium(III) to titanium(IV) and excess H2O2 then
forms an orange/red complex of titanium(IV). I do not know whether this can be isolated as a solid. You could try dissolving some TiCl3 in
concentrated HCl and adding excess H2O2 and then using your vacuum desiccator to dry some of this.
Be careful though with peroxo complexes. Some of these complexes can become dangerously unstable when purified in the dry state and may explode. Not
sure about the titanium(IV) complex, but with peroxo complexes you never know. So, do not make a large amount, try with 1 gram or so.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for the comments Woelen and Clearly.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
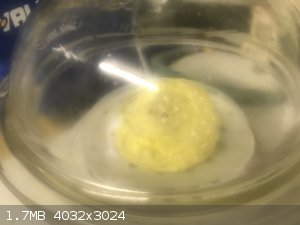
Woelen - I put some of the red complex on a watch glass. See photo. This went into the desiccator under high vacuum where it started bubbling. See
photo. After some 2 hours I came back to find a big yellow dome. See photo. I will leave it in the desiccator overnight to make sure it is dry and
open it in the morning. I wonder what compound this is?
|
|
|
| Pages:
1
2
3
4 |