| Pages:
1
..
37
38
39
40
41
..
44 |
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
Even if it is, it's based on producing an awfully toxic substance - definietly not something you'd like to do on your balcony, and I believe that
would qualify as "needing fumehood".
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I think it is probably something more irritating than toxic, but yes, it requires some well-tested setup, may be the same or next level after
chlorine. So, that's why I proposed acetyl chloride.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Quote: Originally posted by teodor  | | I think it is probably something more irritating than toxic, but yes, it requires some well-tested setup, may be the same or next level after
chlorine. So, that's why I proposed acetyl chloride. |
Are you talking about ketene or acetyl chloride? Because ketene would probably kill you before it irritated you.
[Edited on 10-23-2020 by njl]
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by njl  |
Are you talking about ketene or acetyl chloride? Because ketene would probably kill you before it irritated you.
[Edited on 10-23-2020 by njl] |
I have no experience with ketene, but wikipedia says:
"Exposure to concentrated levels causes humans to experience irritation of body parts such as the eye, nose, throat and lungs. Extended toxicity
testing on mice, rats, guinea pigs and rabbits showed that ten-minute exposures to concentrations of freshly generated ethenone as low as 0.2 mg/liter
(116 ppm) may produce a high percentage of deaths in small animals.
...
An IDLH limit is set at 5 ppm, as this is the lowest concentration productive of a clinically relevant physiologic response in humans.
"
IDLH as 5 ppm is identical to such substances as H2SO4 / SO3 fumes, ozone . For bromine which most of us distil we have 3 ppm.
Comparison with phosgene is not correct because phosgene action is different and delayed.
So, yes, I don't know but it seams no at the same level.
[Edited on 23-10-2020 by teodor]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Because it requires even more equipment? I also left out the nitrogen dioxide route, because it's basically the chlorine/bromine route but
with NO2 instead and no sulfur.
Also the ketene route is flypaper for reckless idiots so I prefer to avoid talking about it as much as possible.
[Edited on 23-10-2020 by clearly_not_atara]
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
Ketene is said to be 8 times more toxict than the all-frightening phosgene.
Ask yourself if you'd be willing to work with 10g of liquid phosgene (that'd be around 0.1 mol, so 2.24dm3? That's almost 2.5 liters of a
deadly gas. Now do the same, but either counting it that the gas is 8 times deadlier or there's 8 times more of it (the second option being actually
more forgiving, as it's easier to notice a leak). Just an analogy that presents how awful ketene is for an inexperienced chemist.
Edit: as a follow up, as I can see some more posts emerged while I was writing this:
https://www.ncbi.nlm.nih.gov/books/NBK224928/
Take a look at this, ketene really IS awful and any underappreciation of it's toxicity comes from, I believe, the scarcity of it's uses. Considering
how much press carbon monoxide or cyanides get, comparing them would be unfair, even though our main hero is much worse.
[Edited on 23-10-2020 by Antigua]
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I will not force anybody to use this substance, just want to point that the link in wikipedia which can proof that is broken, so we don't know.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by teodor  |
I will not force anybody to use this substance, just want to point that the link in wikipedia which can proof that is broken, so we don't know.
|
You've been posting here for more than a year, you've doubtless seen the stupid ketene dance in this thread before, so you should not need me to
traipse through the literature pulling out all of the reasons why underequipped labs should not attempt the prep of ketene. But fine, here:
https://pubs.acs.org/doi/abs/10.1021/ja01216a526
I didn't mention the ketene route because I was responding to a question about small labs using readily accessible chemicals. Ketene is not
appropriate and for that matter it's worse than the others. At least when you gas chlorine you don't do it at 600 C.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Good point, clearly_not_atara. But also the apparatus will never contain much of ketene, in Vogel preparation III,90 the rate of ketene production
with a Ketene lamp was calculated as 0.45 mol/hour. So, probably you can construct it such a way to have only small percentage of internal space be
filled with ketene and make this space really small. Anyway, I don't want to proceed with this. Just pointed that we have no reliable test of
toxicity.
I don't remember really all the dances but now I noticed you mentioned route from S2Cl2 somewere. I have plan to put my hands on this compound. Is
there some route I can check to acetic anhydride from S2Cl2?
(I also don't want to study real toxicity of ketene on humans)
Edit: the good point that ketene route is not as clear and simple as 1,2,3 and it can create also many byproducts.
[Edited on 23-10-2020 by teodor]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
S2Cl2 can be disproportionated with elemental sulfur to form SCl2, which reacts with SO3 to form thionyl chloride, SOCl2. That can be used to
chlorinate acetic acid which can then react with sodium acetate to form acetic anhydride. I believe more direct routes have been mentioned in this
thread which are almost certainly less work.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Acetic Anhydride
A random thought, but would ozonolysis of 2-butyne create acetic anhydride?
https://en.wikipedia.org/wiki/Ozonolysis#Ozonolysis_of_alkyn...
Ozone generators are already made, so only the 2-butyene is a problem. Anyone know how to make 2-butyene?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Maybe taking 2,3 dibromo butane and putting it in Na+NH3 (Makes NaNH2 in-situ) would work?
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
That or the similar synthesis of phenylacetylene via 1,2 dibromoethylbenzene. UC235 has a youtube video following that procedure.
Link: http://orgsyn.org/demo.aspx?prep=CV4P0763
[Edited on 11-5-2020 by njl]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Can you put a link to the procedure that UC235 followed?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It would have to be 1-phenylpropyne in order for cleavage to generate an acetyl anhydride. Phenylacetylene gives you benzoic-formic anhydride.
Probably not practical as alkynes are typically quite hard to make. Unless you can adapt this rxn to OTC:
https://www.organic-chemistry.org/abstracts/lit6/936.shtm
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Could there conceivably be a route to 2-butyne from 2-butanone? In the interest of OTCness.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Exactly how would the 1-phenylpropyne work? A rnx diagram would be helpful. I came up with an idea to make the 2-butyne. Starting with methyl ethyl
ketone, its reduced to 2-butanol. After that, thats dehydrated to 2-butene. This reacts with bromine to make 2,3-dibromobutane. Reaction with Na in
anhydrous liquid NH3 to give 2-butyne. That reacts with ozone to make acetic anhydride. Any problems with this? Also, can I get 2-butanol without
needing to do an expensive NaBH4 reduction?
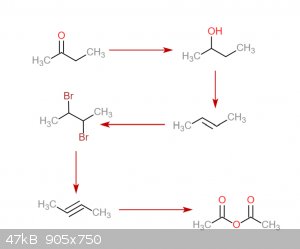
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You actually need to do the double dehydrohalogenation in two steps because otherwise you'll probably get a diamine when the 2,3-dibromobutane
immediately reacts with the NH3 solvent. First remove one bromine with pyridine, then the other with NaNH2. But the other problem is that the
preferred product is the allene, IIRC.
For phenylpropyne the ozonolysis product should be this:
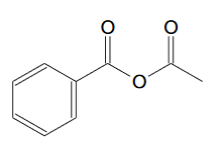
[Edited on 5-11-2020 by clearly_not_atara]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
So how do I convert benzyl-acetic anhydride to acetic anhydride?
|
|
|
Fluorite
Hazard to Others
  
Posts: 138
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Why everyone keeps trying to find a way for acetic anhydride? Just reaction between sodium acetate and disulfur dichloride should make acetic
anhydride ITS SO SIMPLE
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It reacts with (anhydrous) potassium acetate -- the more volatile anhydride distills out. Sodium acetate also works fine but is slightly harder to
dry.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
@Fluorite, a new method can't hurt. The thing I like about this is that there is no need for messy/stinky/hard-to-clean sulfur chlorides.
@clearly_not_atra, do you think that you can try that, my lab is down for the next couple months.
|
|
|
Texium
|
Threads Merged
6-11-2020 at 10:42 |
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Anhydrous Potassium Acetate is more hygroscopic than dry Sodium Acetate.
Chemistry = Chem + is + Try
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Does anyone tried the following reaction?
2Ch3COOH + SbCl3 → (CH3CO)2O + SbOCl + 2HCl
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
A variation on the succinic anhydride method, which was cool, but succinic acid isn't quite as easy to find as... citric acid:
https://en.wikipedia.org/wiki/Itaconic_anhydride
Now, these cyclic anhydrides are obviously more stable than the acyclic ones, so the real idea here is to gas HCl into a solution of the cyclic
anhydride in GAA, which will hopefully open the ring and in this case could hopefully also add to the double bond, destabilizing the ring geometry. In
fact, if HCl won't do it, give HBr a shot, it's a million times stronger.
Once you generate the open-chain acyl halides, you should get a reasonable equilibrium with the acetyl halide. Then AcCl (bp 52 C) or AcBr (bp 76 C)
should be able to distill out. (This method really makes AcCl or AcBr, but it is not too hard to obtain Ac2O, and anyway you can often use any of the
three with slightly altered methodology.)
____________________________________________________
Mind has been on anhydride lately for some reason. Anyway, Orgsyn mentions that diphenyldichloromethane can be used to dehydrate sodium benzoate to
benzoic anhydride:
http://orgsyn.org/demo.aspx?prep=cv1p0091
http://en.wikipedia.org/wiki/Diphenyldichloromethane
This can be regenerated from the benzophenone byproduct by:
- reduction to the alcohol (Zn/NaOH)
- HCl Sn2
- free-radical chlorination with TCCA in benzene or similar
This is all very tedious, but it's also the first OTC method not involving gassing that seems to have any chance of working, which is mildly
interesting.
[Edited on 16-12-2020 by clearly_not_atara]
|
|
|
| Pages:
1
..
37
38
39
40
41
..
44 |