| Pages:
1
..
36
37
38
39
40 |
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
if you heat the seals on those cans to much they do start to leak, so you might want to keep an eye on it. If the temp is <100C you should be right
though
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
You might also want to put two layers of gauze and keeping it wet with ice cold water, being careful not to let it drip into the receiver.
Dichloromethane is not something one should distill with and air condenser. It's extremely volatile. The losses are great, and the vapor toxicity is
pretty serious.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
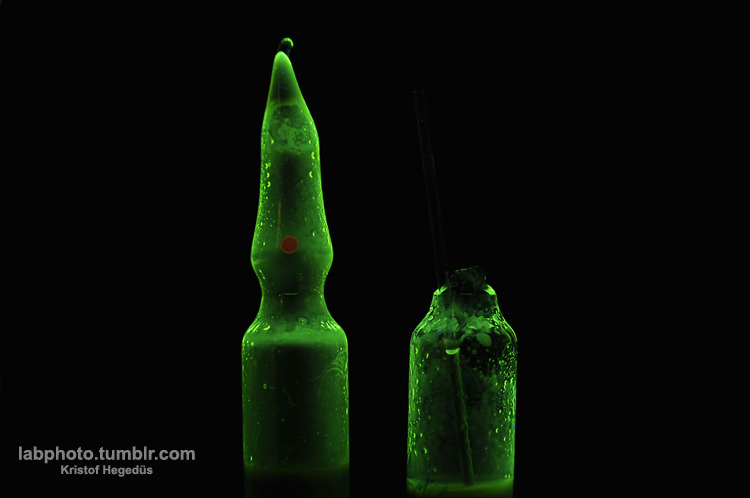
I have done 5x2 parallel reactions in ampoules on a 0,5mmol (170mg) scale to see that happens if I use different solvents.
This one was in dimethyl-sulfoxide, a really universal and great solvent and this one was the only what contained only one product. The pictures was
taken under UV light, this is why it looks so fantastic.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Boron Trioxide
Harmless

Posts: 42
Registered: 18-6-2012
Member Is Offline
Mood: No Mood
|
|
Here is a macro picture of the surface of a soon to be tested magnetite anode

|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Want to see some GLASS BLOWING PORN? Check out this youtube channel I found
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I just can't help myself. This one has me drooling with avarice.
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=15107...
|
|
|
ElectroWin
Hazard to Others
  
Posts: 224
Registered: 5-3-2011
Member Is Offline
Mood: No Mood
|
|
the buyer got a great deal on that one
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Reserve was never met. I could never get him to tell me how much he wanted for it.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Not too much of a surprise -- Ir (99%) prices have been dropping from the beginning of this year from around $1000 to $850 per troy ounce (31.1034768
g), meaning that this ingot containing 458.901g of Ir next to some gold, is worth around $12k5 of Ir alone.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Until, you take into account that iridium prices HAVE been dropping since the first of the year and that is still in "mine bar" state. It would have
to be separated and purified before you could even come close to spot price and then you still wouldn't get that price for it.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Fair enough, but do you expect to get a "mine bar" at 10% of the spot price? To me that would sound like an unrealistic bargain.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Just some methyl orange... Destined to become p-aminodimethylaniline

|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Nice bottle! How much of that ¼lb is left?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
It's more than 3/4 full- hence why I decided to try the reduction in vogels 5th : )
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Magnetite  ? Isn't this just a pitted piece of SS? If not - how you made that? ? Isn't this just a pitted piece of SS? If not - how you made that?
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Heres a couple of my own:
HNO3 condensing, the flask it was distilling from halfway through cooling, some CuSO4 crystals (longest is about 5cm) and finally, about 5g of
nitroglycerin detonating under a pile of half-burnt thermite.
Also, I don't know how to embed images, and haven't seen a thread on how to do so. help please!
   
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Attaching images is preferred, at least while the /scipics/ FTP is down.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Found a piece of calcite!! There were hundreds of small chunks but this was the biggest one I could carry home. One piece was the size of a large air
conditioner!
 
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>Mailinmypocket</strong>, I am so mad at you for not carrying the air conditioner sized block home and shipping it to me!  And I thought we were friends. And I thought we were friends. 
Where did you find such massive crystals? If you can get more, I'd be very very very intersted. PM me, with
details, please.
[edit] Also, any chance you could supply a high resolution photo of that thing in full sunlight with a neutral background? That would make a damn
nice desktop background. Of, if you have a UV lamp, test that sucker for <a
href="viewthread.php?tid=14644&page=24#pid266048">fluorescence/phosphorescence</a>. Too bad it's not halite, then you could cleave and
polish some salt plates for IR-spec.
[Edited on 7/27/13 by bfesser]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Wow, that thing probably has fluorescent zones. It would be cool to see how they spread...
I hope you tried to find more transparent ones in the pile of smaller minerals. These things have interesting optical properties. Be sure to check it
with a polarizer. Ask if you don't know how.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Come tomorrow when the sun is shining in my place I will for sure take a nice picture of the big specimen on a neutral background. I found the
crystals at an abandoned mine near Ottawa, Canada called "O'Brien mine". Google finds nothing related to this place though, it might be named after
the owner of the abandoned property- I don't know. It used to be a feldspar mine and there are massive piles of the stuff everywhere, they make
incredible yellow sparks when struck together! The calcite was found in a hole amongst some very compact sand... Digging in the hillside in that area
revealed more and more. I was only able to bring what I could stuff in my pockets and carry in one hand (the large piece) as I was on a bike. Tonight
I will take some pictures under UV.
Endimion, I would be interested in taking some photos with a polarizer- not sure if I have what is needed but ill try it out if I do!
They really are beautiful though 
   
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mailinmypocket  | I found the crystals at an abandoned mine near Ottawa, Canada called "O'Brien mine".
. . .
Endimion, I would be interested in taking some photos with a polarizer- not sure if I have what is needed but ill try it out if I do!
|
I'm literally salivating while looking at these photos. If I'm ever in Ottawa, could you serve as a local
guide to this mine? Do you happen to know who owns the property?
If you don't have a polarizing filter, just smash open your LCD monitor and peel one off. Then just slap it over your camera lens, and
<em>bingo presto</em>, polarizing filter!
[edit] Almost forgot this! <a href="http://en.wikipedia.org/wiki/Birefringence" target="_blank">Birefringence</a> <img
src="../scipics/_wiki.png" /> will blow your mind.
[Edited on 27.7.13 by bfesser]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Of course, provided you don't mind biking! The mine itself is extremely old and I have no clue who owns the property. The path to get there is
essentially an old horse and wagon trail, grown in with small trees and whatnot- impossible to drive a vehicle on. Next time I will take some photos,
it is beautiful in the summer provided you stay clear of the "bottomless pits of death", in the winter they fill with water and freeze... Just an idea
of it, in the winter(photo below). These are friends of mine btw, not me. I have an old LCD monitor but I'm hesitant to tear it apart... Hoarding at
its best 

|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
I think LCD monitors have somewhat blurry filters that show clear background only when something is directly behind them.
Polarizing filters can be found for a couple of euros online and are transparent. Every chemist should have one. You can use linear or circular
polarizers, it doesn't matter, but you have to check the proper side for the CPL.
You can put an object between two crossed polarizers, but then you can't look at anything larger than the polarizers themselves. The solution is to
use a polarized light source - an LCD screen. Use a pure white photo file or something, then stretch it to fullscreen, place the object between your
camera/eye and the screen, and put a CPL filter on your objective/eye and turn it until you make the background light disappear.
If you know the basics of stereochemistry, you know what I'm talking about.
This is essentially a photoelasticity test. Your calcite should look very different. Try to rotate it and you'll see how parts of it change
transparency.
|
|
|
bfesser
|
Thread Moved
29-7-2013 at 18:10 |
Zwitteron
Harmless

Posts: 6
Registered: 16-6-2013
Member Is Offline
Mood: No Mood
|
|
Today i just made nice new coating for batery cover on my HTC HD2. Cover was from steel (aproxx 78iron, 18chromium, and tiny bits of other).
It was made in solution of 200g copper sulfate, 50g conc sulfuric acid and 0,4g thiourea.
Current 2 amps from regulated source for 20 minutes (voltage was around 1,3).
It isnt as shiny as i wish, but i would try some very fine sand paper.
<a href="http://imageshack.us/photo/my-images/812/f6kv.jpg/" target="_blank"><img src="http://imageshack.us/a/img812/6812/f6kv.jpg"
width="500" /></a>
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: fixed
broken image(s)]
[Edited on 30.7.13 by bfesser]
|
|
|
| Pages:
1
..
36
37
38
39
40 |