| Pages:
1
..
32
33
34
35
36
..
77 |
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by alexleyenda  |
The procedure could not be more simple, PDC with the molecule to oxidize in a solvent, the junk is probably chromium junk. |
Was it 'for your education', or for fun?
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
For my education and for research !
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice. Where do you research/for who?
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
At the university of montreal.
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
CuCl2 which will be used for woelen's CsCuCl3 synthesis 

|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice, that's a great pic! Post your prep of the second compound when you get a chance.
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Tungsten trioxide powder.it is more yellow than I'd photographed.
[Edited on 11-7-2015 by sasan]

|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
And this is the picture of W3O8 green powder.another oxide of tungsten

|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
menthol crystals C10H20O
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexanol

|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Hexamine nickel chloride NiCl2.6 nh3
 
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Copper ammonium sulfate crystal

|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Manganese ammonium sulfate crystals.

|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Potassium tetracyanonickelate(ll).

|
|
|
Starcruiser
Harmless

Posts: 18
Registered: 11-7-2015
Location: Orion Arm/Milky Way
Member Is Offline
Mood: No Mood
|
|
My crowded little fumehood 
Apparatus for making some ammonia soln. (NH4NO3 + KOH), improvised scrubbers in the background - some 30 years old milk bottles filled with water,
suck-back traps in between (probably unnecessary - I`ve also provided some aquarium anti suck-back valves - these work like a charm (but I don`t rely
on them if I were generating NO2 or something else more corrosive).

[Edited on 11-7-2015 by Starcruiser]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Looks great !
How do you get it so white and clean ?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
My fumehood was that clean right after I built it.
It would be very hard to get that way again (it would probably take a repainting).
|
|
|
Starcruiser
Harmless

Posts: 18
Registered: 11-7-2015
Location: Orion Arm/Milky Way
Member Is Offline
Mood: No Mood
|
|
Thanks aga.
It is rarely used (unfortunately !) and it is only about 1 year old. I work slow, prepare a lot before actually do any experiment (this setup, for
example, took couple of weeks to put together).
Not so much work on the "fiery side" of the hobby, also trying to avoid as much as possible visible / smelly fumes (very busy apartment neighborhood,
low profile is mandatory - afraid of "legal" interference).
And, yes, I am kind of obsessed with cleanliness (my wife too).
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
In regard to a request by aga in this thread, here are some pictures pertaining to what I have done this month so far.
I thought I would post them here since most of them are quite pretty and that way I don't have to double post them.
1: My Bromine distillation setup (it was very cold that day, hence no condenser)
2: My yield of Bromine
3: Cobalt(II) Chloride (almost anhydrous)
4: Cobalt(II) Nitrate Hexahydrate
5: Cobalt(II) Nitrate Hexahydrate after crystallization in a petri dish
6: Praseodymium(III) Nitrate (hydrated)
7: Sodium Ruthenate(VI)
8: Top layer = 2-Ethylhexanol, Bottom layer = Aqueous Sodium Phthalate
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Damn those are nice pictures! Nice experimenting.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Come on TAS, give diddi some credit too! 
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Oh, alright then. diddi did a little bit. (and by that I mean he helped a lot. Thanks diddi!)
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
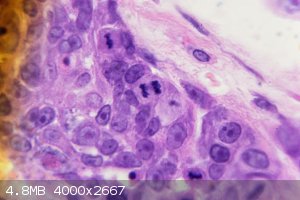
Mitotic skin cancer cells under my microscope at 1000x. You can clearly see the cells at different points in mitosis. The most incredible is a cell
near the middle of the picture in anaphase. The spindle fibers are clearly visible! The yellowish tint near the edge is due to my camera not being
perfectly in line with the microscope.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Cool! What stain is that? How is the sample prepared?
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
It was an H&E stain. That stain is really good for viewing cancer cells since the basic haemotoxylin used binds to acidic DNA/RNA. This makes the
extreme amounts of mitosis happening more visible and easier to identify.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
You don't have skin cancer, do you?
Sasan and TAS, nice pictures! How's you make ethylhexanol? I'd like to!
|
|
|
| Pages:
1
..
32
33
34
35
36
..
77 |