| Pages:
1
..
29
30
31
32
33 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Hmm. A week should be almost enough time to understand the reaction mechanisms !
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Grignard reagents and reactions:
This post is the follow up to Carbon atoms as Lewis bases, please refresh as what follows might not make a lot of sense otherwise.
Grignard reactions make use of organo-metallic compounds known as Grignard reagents, obtained by reacting an organo-halide with magnesium metal. The
resulting complex R-CH<sub>2</sub>-Mg-X carries a partial negative charge on the Mg bonded C atom due to the strong difference in
electronegativity between C and Mg, making it a strong nucleophile (Lewis base).
Below’s an example of the full sequence (three distinct steps) of a Grignard reaction between 1-bromopropane, Mg and pentan-2-one:
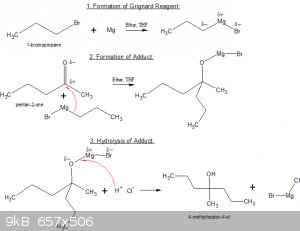
Step 1: formation of the Grignard reagent.
1-bromopropane is slowly added to a suspension of Mg shavings in super-dry ether of THF. The organo-magnesium complex forms.
Step 2: formation of the Adduct.
pentan-2-one is added to the solution of Grignard reagent. The partially negatively charged C-Mg atom bonds to the partially positively charged C atom
of the carbonyl >C=O group. The partially positively charged Mg atom bonds to the O atom in the carbonyl group.
The reaction between two carbon atoms (one as Lewis base, one as Lewis acid) is often called a C-C coupling reaction or chain extending reaction.
Step 3: hydrolysis of the Adduct.
The adduct is rarely the desired end-product. Usually and equivalent of dilute HCl solution is added, which converts the adduct to alcohol by
electrophylic attack of H<sup>+</sup> on the Mg-O bond.
After work up, the end product 4-methylheptan-4-ol is then obtained.
Carbonyl groups are very suited as Grignard substrates because of the strong polarisation of the C=O bond, as shown here.
As regards the organo-halide starting point, suitability depends on choice of halogen. In order of suitability: I>Br>Cl>F.
Water is the enemy of this reaction, as water hydrolyses the Grignard reagent. All reagents used must therefore be of suitable dryness.
[Edited on 2-5-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Perhaps a week was being optimistic ...
So, first off we got the formation of the Grignard reagent.
Electronegativites of Mg =1.3, C=2.6, Br=3.0
The Br atom and the adjacent C fight over the weaker Mg and rip the s**t out of it, leaving that set of 3 atoms (the functional group ?) unstable, in
that the group are electron deficient.
Reading up (in this thread) why the C ends up being a strong Lewis Base instead of the Mg or Br ...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | [...] in that the group are electron deficient.
Reading up (in this thread) why the C ends up being a strong Lewis Base instead of the Mg or Br ...
|
It's simpler to merely consider that the electron density (ψ2 is the light green 'bell', remember that?  ) of the Mg-C bond is skewed toward the C-atom (the picture is a schematic and not to
any scale): ) of the Mg-C bond is skewed toward the C-atom (the picture is a schematic and not to
any scale):
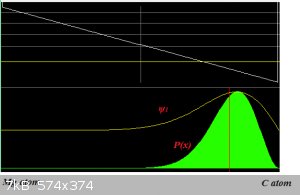
The C-atom therefore carries a partial negative charge.
[Edited on 2-5-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The electron density probability plot is pretty much in the old noggin since the first time.
An amazing feat of Teaching, considering the density of this noggin.
Inescapable is the Br's superior electronegativity, which is what makes me wonder why It is not the 'active' component instead of the C.
I get the feeling that it is an accomplice to a far more complex mechanism that a noob like me should not be asking about in public.
[Edited on 2-5-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Inescapable is the Br's superior electronegativity, which is what makes me wonder why It is not the 'active' component instead of the C.
|
Good question but easily answered. Br's electronegativity means that it won't share another of its three remaining unbonded MOs with any Lewis acid.
So structures like -Mg-Br-C- can never arise. Br- is an extremely weak Lewis base.
See also HBr:
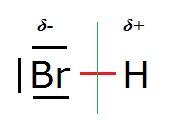
One could imagine a [H-Br-H]+ ('bromonium cation') structure but in reality that doesn't exist (bar perhaps in extreme acid
conditions).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. Looking at the orbitals and trying to relate that to Lewis dots, it appears to me like this :-
The Mg - Br bond is a σsp effectively filling Br's p orbitals.
The Mg - C bond is also σsp however C's p orbitals are still incomplete, therefore less stable, so it's -ve charge makes it more
nucleophilic than the -ve charge on the more stable Br electron configuration.
Add to that the higher negativity of Br and it is less likely to let go of any electrons than either C or Mg.
Mg has no more 'easy' electrons to share in bonding, which leaves C as the atom most likely to engage in further bonding, making it the 'active' bit.
Poor old Mg has been robbed down to a slight +ve charge, and is so depressed it's unwilling to even talk about it.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Just keep asking questions until everything makes sense. We are more than happy to share our knowledge with you. I'll also add some stuff on Grignards
to further supplement blog's post. For consistency, I'll also use 1-bromopropane and pentan-2-one.
| Quote: | | So, first off we got the formation of the Grignard reagent. |
Formation of Grignard reagents are thought to proceed through a SET (single-electron transfer) mechanism that takes place on the surface of the
magnesium metal. For the sake of simplicity, you can envision this reaction as consisting of three steps: oxidation of the magnesium metal, reduction
of the organic halide, and formation of the R-MgX complex:
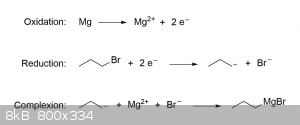
For the purposes of preparing a Grignard reagent as part of your next practical, the above explanation should be more than sufficient; however, if you
would like a slightly more advanced understanding of what is going on, we can further break the reaction down to elementary steps and intermediates.
While the true mechanism for the formation of Grignard reagents isn't actually known (particularly the mechanism of the halide reduction step), the
reaction most likely involves an initial electron transfer from the magnesium metal to the organic halide (R-X) to give a radical anion
(R−X−•) and a magnesium(I) cation (Mg+).
The unstable radical anion then collapses into an organic radical (R•) and a halide anion (X−), the latter of which serves as the new
counterion for the magnesium(I) ion (Mg+X−). The magnesium(I) ion then transfers a second electron to the organic radical to
give a carbanion (R−) and a magnesium(II) ion (Mg2+), which together with the halide anion make up the organometallic complex
known as the Grignard reagent (R-MgX).
The mechanism described above can be conceptualized as follows:

Recall that arrows with a "fishhook" on the end depict the transfer of a single electron, while the more common "full" arrowhead represents the
transfer of a pair of electrons. Also, remember that a radical is where an atom has an unpaired valence electron. In other words, one of the occupied
valence orbitals only has one electron in it (called a singly occupied molecular orbital, or "SOMO" for short). This typically makes radical species
extremely reactive. But don't beat yourself up if you don't completely understand everything discussed above, especially the SET stuff, as it isn't
exactly introductory-level material.
| Quote: | | Reading up (in this thread) why the C ends up being a strong Lewis Base instead of the Mg or Br ... |
As blogfast already pointed out, most of the electron density in the C-Mg bond is shifted towards the carbon. But something else to consider, which
hasn't been mentioned yet, is the leaving group you'd get if Br− attacked the carbonyl group. Think about it: why is it that when you add
concentrated HBr or a bromide salt to a solution of acetone, you don't end up with this (at least not in any significant quantity):
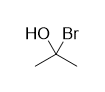
Surely the nucleophilic bromide ions will try to attack the electrophilic carbonyl carbon, right? And if the solution is strongly acidic, the oxygen
on acetone will also be protonated, making the carbonyl carbon even more electrophilic than it normally would be. So why don't we get the molecule
shown above?
The reason is primarily due to the unusual instability of geminal halohydrins. Because the carbon is bound to two extremely electronegative groups, it
is highly electron-deficient and will immediately attract any nearby electrons. Since the oxygen has an extra pair of electrons that it can form bonds
with, and the bromide ion makes a rather good leaving group (i.e. it isn't very strongly attracted back to the carbon as it leaves), the oxygen will
just donate a pair of electrons to the electron-deficient carbon and kick out bromine, returning the unfavorable geminal halohydrin back to a much
more favorable carbonyl configuration.
Also, if the media is strongly basic, like in Grignard reactions, the oxygen will be deprotonated and carry a negative charge, meaning TWO free lone
pairs available to donate to carbon. This makes the elimination of bromine in order to return to a neutral and more stable configuration even MORE
favorable. With carbon as the attacking nucleophile, however, elimination in this manner cannot happen, as the leaving group (a negatively-charged
carbon atom) is way too strongly attracted back to the electrophilic carbon to leave.
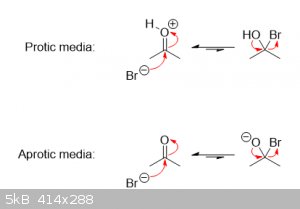
| Quote: | | Inescapable is the Br's superior electronegativity, which is what makes me wonder why It is not the 'active' component instead of the C.
|
There's also a flip-side to that as well: the more electronegative an atom is, the less it will want to share its electrons should the opportunity
arise. That's one of the reasons ions like F− and Cl− are such weak bases.
| Quote: | | I get the feeling that it is an accomplice to a far more complex mechanism that a noob like me should not be asking about in public.
|
Now you've gone and opened Pandora's box. While Grignard reagents are usually taught at the introductory level as simply being this R-MgX complex
floating around in some ethereal solvent (usually diethyl ether or THF), the truth is that they actually exist as tetrahedral adducts with the solvent
(which is why they dissolve in it). The reason ethers are so commonly used as solvents in Grignard reactions is because not only are they unreactive
towards the Grignard reagent, but they also stabilize the R-MgX complex by coordinating with the magnesium atom and giving it a full octet:
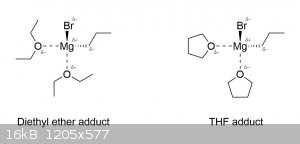
Notice that the magnesium is surrounded by electronegative atoms, which provides a sort of stabilizing effect. Similarly, the Grignard reagent also
coordinates with the carbonyl compound to form a six-membered cyclic ring, which may also help to explain why bromine isn't the active attacker. It is
really through this structure that most Grignard reactions are thought to actually proceed, though the mechanism is often simplified when first
introduced at the lower levels. I haven't done a great deal of research regarding the specifics of these Grignard reaction mechanisms, but my guess is
that the electrons move like this during the attack on the carbonyl carbon:
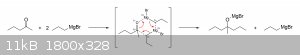
Anyway, I hope this has been of at least some help to you. Don't be discouraged if some of this seems a little overwhelming. The level of depth gone
into here far exceeds what is typically taught in introductory-level courses, so don't be put off of trying to learn organic chemistry because of it.
And don't be afraid to continue to ask questions if you're still not clear on anything.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
That was very interesting, Darkstar. Thanks.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
discouraged ? No way !
This is cool stuff, and the greater the depth of my confusion and ignorance the better !
To be honest, it's all making more sense now than it did in the lesson before calculus class started.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
@darkstar: "coordinating with the magnesium atom and giving it a full octet"
What does 'co-ordinating' mean ?
if it is not forming bonds then erm, what ?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
The oxygens on the ether/THF molecules are forming bonds with the magnesium atom. "Coordination" just means that both electrons of the bond
come from the same atom. In this case, both electrons of the O-Mg bonds come from oxygen, which are dropped into empty orbitals on magnesium.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ooooh ! the Empty Orbitals thing again.
So the co-ordinating O just jumps in and lobs an electron-filled orbital at Mg, which is so happy to see electrons again that it just 'has it'.
Now there's a question : in this case, would the Mg do any hybridisation of the remaining two electrons in the s orbital ?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Seeing as the Dark Lord is back, how about an actual do-able OC synth that demonstrates an OC mechanism/principle ?
Got loads of reagents now 
Edit:
And stacks of 250ml beakers for some reason.
[Edited on 3-9-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Seeing as the Dark Lord is back, how about an actual do-able OC synth that demonstrates an OC mechanism/principle ?
Got loads of reagents now 
|
It's been said before but I'll say it again: a decent Fischer esterification is a very doable OC synth with a multi-step reaction mechanism.
Doable but not entirely easy either.
It does require a pure short chain fatty acid.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Bring it on ! (had to google what "short chain fatty acid" is)
I got glacial acetic acid and sodium formate.
Butyric acid may be available if i cap, shake and vomit (i tend to vent frequently anyway).
What other reagents needed ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A short alcohol: propanol or butanol, e.g. Ethanol or methanol work too but a bit boring, really.
> 95 w% H2SO4 (catalyst).
Look up a procedure on orgsyn (or similar) and adapt it.
[Edited on 3-9-2016 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
As a reminder, I made a post a while back discussing some of the basic theory behind the mechanism of the Fischer esterification. Maybe re-read that post if you do decide to try one. We can always go back over anything that you still
aren't clear on.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Checking at the back of all cupboards reveals no butanol or propanol.
Ethanol there is aplenty, also Butane.
Would it be too boring with ethanol ?
Edit:
Maybe boring, but at least i know what ethyl acetate smells like, plus it follows the mechanism as Darkstar kindly posted, and provided a link to
above.
[Edited on 4-9-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'm considering reviving this thread, by revisiting and refreshing some core concepts of QM/QC, while keeping it Lite.
Concepts under consideration are:
* Complex numbers
* Functions as vectors and as matrices
* The TDSE and TISE
* Quantum operators
* Measurement and mean values of observables
Let me know if there's any appetite for it.
Thanks.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sorry i'm late.
Has class started yet ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nope but we can start on the morrow, if it pleases you.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Complex numbers (recap)
Complex numbers (aka imaginary numbers) arise because when we ask "What's the square root of a (a Real number)?", then if
the answer is b, then:
√a=b⟺b2=aif (a∈R)
As the square of a Real number is always positive, we can't take the square root of a negative Real number.
1. The imaginary number i:
We define:
i=√−1
Then, with a a Real number:
√−a2=√a2×−1=a√−1=ai
And:
(ai)2=a2i2=a2×(√−1)2=a2×(−1)=−a2
2. Composite complex numbers:
Most (but not all) complex numbers have a Real and a Complex part, where Re is the Real part and Im is the
Complex part:
c=Re+Im×i=x+yi
For example:
−5+3i
We say that c is a member ("an element") of the group of Complex numbers:
c∈C
3. Representation in the Complex space:
The Complex number as a vector:
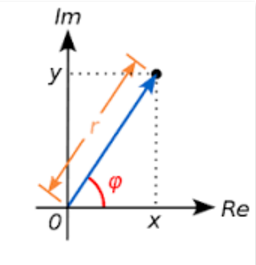
Algebraically:
c=rcosφ+risinφ
(rcosφ)2+(rsinφ)2=r2cos2φ+r2sin2φ
And because, always:
cos2φ+sin2φ=1
⟹(rcosφ)2+(rsinφ)2=r2(cos2φ+sin2φ)=r2
And because:
Re=rcosφand Im=rsinφ
⟹r2=Re2+Im2=x2+y2
The value r:
r=√x2+y2
... is often called the modulus of the complex number.
Exercise:
If:
r2=12
And:
φ=π4
Then write the complex number as a + bi.
Next up: complex conjugation > basic operations with complex numbers > ...
[Edited on 1-11-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Gulp.
I forsee disturbing dreams where a guy from Trinidad comes up to me and says :
" i is your imaginary friend "
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
We've seen all this above, it's just presented more beautifully.
So if earlier you were only physically present or were really daydreaming about girls with really big t*ts, now's your chance!
 
|
|
|
| Pages:
1
..
29
30
31
32
33 |