| Pages:
1
..
29
30
31
32
33
..
77 |
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Bezaleel  |
Interesting to see that this is a purple compound, as cyanuric acid and copper(II) also combine to form a purple compound. So, the purple colour may
well arise from the N-Cu bond, when N is also bonded to C. |
Not surprising, actually- most amine-type copper(II) complexes are purple or blue-purple, [Cu(NH3)4]2+ being the prime example. The copper cyanide,
however is a bit more surprising, as cyanide is a very strong-field ligand, and you'd expect a different colour.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by blargish  | Not the best quality pic in the world, but a cool effect seen when dark-brown crystals of potassium tetraperoxochromate(V) are put into hydrochloric
acid, with the formation of a blue chromium oxide diperoxide complex: CrO(O2)2.
|
Is the second complex isolate-able? Nice picture! SuperVillan, nice picture too!
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Here's a couple I made with pictures taken via scanning electron microscopy, and colorized using GIMP and public-domain images from Wikimedia Commons.
I entered an art contest with them at the university I work at ... didn't win, but they're decorating one of the chemistry buildings!
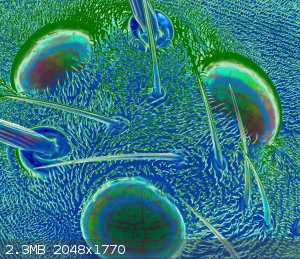 "Dorsal Ocelli"
"Dorsal Ocelli"
Insects are famous for their compound eyes; who hasn’t seen a microphotograph of the honeycombed lenses of an ant’s eye? Less well known is the
fact that many insects also have simple eyes on the tops of their heads. Pictured here are the simple eyes of a fruit fly. What do they see? Light?
Motion? Shape? What would it be like for humans to have two distinct forms of sight?
 “Are Lovecraftian Horrors Circling Your Bananas?”
“Are Lovecraftian Horrors Circling Your Bananas?”
Humans seem to have a kind of common sense, an intuition about how the world aroundthem looks and works. But because it's been built around what is
common in human experience, it can lead to counterintuitive results on small spatial scales (quantum mechanics) or at very high velocities
(relativity). Here's a less extreme example: this is the mouth of a fruit fly. Is this what you'd naturally associate with your household insect
friends?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice! What method did you use to colorize them?
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Some pictures of the slow precipitation of Lead(II) Iodide by heating the solution to 95C so all the compound dissolves and slowly cooling down again.
Pictures of dry crystals in vial soon to follow.
  
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
blargish
Hazard to Others
  
Posts: 166
Registered: 25-9-2013
Location: Canada
Member Is Offline
Mood: Mode Push
|
|
Quote: Originally posted by TheAustralianScientist  | Some pictures of the slow precipitation of Lead(II) Iodide by heating the solution to 95C so all the compound dissolves and slowly cooling down again.
Pictures of dry crystals in vial soon to follow. |
Just got some lead(II) nitrate and can't wait to try this reaction out! It looks awesome!
BLaRgISH
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Yes, it is a beautiful process to watch.
It looks like there is so much product there, but when dry all that barely fills up a 4mL vial.
I also love how the yellow precipitate dissolves to give a colourless solution.
[Edit] Typo.
[Edited on 24-4-2015 by TheAustralianScientist]
[Edited on 24-4-2015 by TheAustralianScientist]
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Part of it was using the Color Balance and Colorize tools. For the first, I used the clone tool to copy image info from other pictures (eg, a CD which
had been microwaved, a butterly's wing under the microscope). I used the "color" setting, meaning that the patterns of saturation and value are
preserved, but the color is carried over. For the second, I made a transparent layer over the main image which had the edges brought out and then
motion blurred and colorized.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Gleb
Harmless

Posts: 8
Registered: 23-4-2015
Location: Russia
Member Is Offline
Mood: No Mood
|
|
My crystals
      
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Beautiful pictures, need details!
|
|
|
Gleb
Harmless

Posts: 8
Registered: 23-4-2015
Location: Russia
Member Is Offline
Mood: No Mood
|
|
My crystals 2
    
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
Gleb, truly beautiful crystal specimens!
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Small (0.5cm) crystals of Neodymium sulphate.

If you're not part of the solution, you're part of the precipitate.
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Gleb, those are beautiful. What's your secret?
Also Nezza, that is awesome! I've never seen neodymium salts before.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
There's several in "The Trouble with Neodymium..." thread! The ones I've produced all share the characteristic that they have different colors under
different lighting (sunlight, CFL, etc.). Very cool!
Nezza, those are fantastic crystals. How big are they?
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Indeed, Gleb, how'd you grow those? They appear to not have been suspended by string...?
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Gleb, you can't leave us hanging like this! At least tell us what compound the crystals are made of!
|
|
|
Gleb
Harmless

Posts: 8
Registered: 23-4-2015
Location: Russia
Member Is Offline
Mood: No Mood
|
|
1 and 2 double copper-calcium acetate CaCu(CH3COO)4*2H2O
3 yellow blood salt K4[Fe(CN)6]*3H2O
4 nickel sulfate NiSO4*6H2O
5 red blood salt K3[Fe(CN)6]*3H2O
6 potassium dichromate K2Cr2O7
7 iron sulfate Fe(SO4)*7H2O
8 nickel sulfate NiSO4*7H2O (7H2O not 6H2O)
9 Rochelle salt KNaC4H4O6*4H2O
10 copper acetate Cu(CH3COO)2
11 potassium chromate K2CrO4
12 magnesium sulfate MgSO4*7H2O
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Amos  | | Gleb, you can't leave us hanging like this! At least tell us what compound the crystals are made of! |
But Amos, from the looks of it, they weren't hanging... 
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Here is some Bismuth metal I extracted from Pepto Bismol, mixed with a bit of methanol
   
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
This is the best thread on the site. Perhaps my favorite on the web.
You guys really know how to rock!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
How did you reduce the bismuth subsalicylate? Hydrochloric acid and aluminum, perhaps? I have read of people attempting to reduce it with carbon, but
reported poor results.
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Quote: Originally posted by Loptr  |
How did you reduce the bismuth subsalicylate? Hydrochloric acid and aluminum, perhaps? I have read of people attempting to reduce it with carbon, but
reported poor results. |
That is exactly what I did. I then used some methanol to rinse the salicylic acid out
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I've tried that one and it yields Bi powder, which isn't very interesting to me so I tried melting it down to an ingot. That failed, and instead it
oxidized to yellow bismuth trioxide! Too finely powdered, I guess.
|
|
|
Gleb
Harmless

Posts: 8
Registered: 23-4-2015
Location: Russia
Member Is Offline
Mood: No Mood
|
|
Photoluminophor
Combining photoluminophor, epoxy glue and bottles of brandy.
  
|
|
|
| Pages:
1
..
29
30
31
32
33
..
77 |