| Pages:
1
..
28
29
30
31
32
..
40 |
Boffis
International Hazard
    
Posts: 1903
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@DJF96
I’m not sure that they are polymorphs, for various reasons I think their formation is pH or perhaps temperature dependent. The former is based on
the differing reactions between acetates and chlorides of the same metal; violuric acid is a weak acid (pK 4.70) but slightly stronger than acetic
acid (pK 4.76) but much weaker than hydrochloric acid. I intend to investigate this with various organic buffers (to avoid adding ammonia or alkali
metals since they react too) and ion exchange resins.
When I did the initial work many years ago I started in an unheat lab in winter at about 8 C, subsequent work was all done at normal room temperature.
A third facture may be purity of reagent, which I am currently investigating, because I made the early batches in small quantities and each time used
a different method. A possible side product is 5-nitrobarbituric acid which forms sparingly soluble salts but I have no data on their colours.
Violuric acid forms both salts and chelates; alkalis and alkaline earths + some organic bases form salts of the nitroso tautomer while Fe and Co form
extremely soluble chelates (Fe intense deep blue and Co deep orange) (eg Leermakers & Hoffman JACS 1958 p5663). Most of the post 1800 work deals
with the chelates and almost nothing appears in relation to the salts. Mn2+, Zn2+, Cd2+ and UO22+ all resemble alkaline earth metals in the way they
react. Cu2+ appears to form a sparingly soluble olive green chelate.
So there are lots of possibilities and I could write a book on this fascinating compound and is numerous close relatives! When I get more time in the
lab I’ll investigate further and report.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Working with trinitrobenzene-Li salt what has a strong fluorescence under UV:

And my gloves after I have made Nurd Rage's blue smash glow crystals in a flask that accidentally cracked in my hands:

I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Zan Divine
Hazard to Others
  
Posts: 170
Registered: 3-12-2011
Location: New York
Member Is Offline
Mood: Wishing all the worst that life has to offer to that SOB Wayne Lapierre
|
|
I've posted some K & Na ampoules before. The ones I'm making for my present client are bigger. The below is sodium (ca. 1/2 lb.), for comparison
is 15 lb. Marvin.
Attachment: phpIruPfF (62kB)
This file has been downloaded 1237 times Attachment: phplTxDXQ (90kB)
This file has been downloaded 1199 times
[Edited on 2/11/2013 by Zan Divine]
He who makes a beast of himself gets rid of some of the pain of being a man. --HST
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
holy crap! that's amazing!!
all above information is intellectual property of Pyro.  |
|
|
elementcollector1
International Hazard
    
Posts: 2689
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
How do you DO that?!
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I have a special place in my heart for molybdenum … right after copper. Could you please post some details on this in another thread? Or
if I just plain missed it, could you please PM me with the TID?
|
|
|
Zan Divine
Hazard to Others
  
Posts: 170
Registered: 3-12-2011
Location: New York
Member Is Offline
Mood: Wishing all the worst that life has to offer to that SOB Wayne Lapierre
|
|
Conceptually, it's actually easier than the method I described for the 3/4 " tubes last summer. The glass is 1.5" in diameter and is really more than
a single flame torch (what I have) can handle easily.
Consequently, my constricted areas are very short and tricky to seal.
The method is simple, devil is in the details. Long glass tube, seal one end, make a constriction where you want the ampoule to end. Add coarse SS
wool ball above constriction and balls of OIL FREE metal above that. Attach vacuum pump. Tilt tube at 45 degrees, wrap with heating tape. Melt metal.
It flows into the ampoule which is then torch sealed.
Any oil you miss makes the tube cloudy. Molten metal continuously flowing over constriction tries to wet it making sealing doubly tricky.
[Edited on 2/13/2013 by Zan Divine]
He who makes a beast of himself gets rid of some of the pain of being a man. --HST
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bfesser  |
I have a special place in my heart for molybdenum … right after copper. Could you please post some details on this in another thread? Or
if I just plain missed it, could you please PM me with the TID? |
Sure! Ill just post it here. I meant to show the final product but I forgot, so I dug it out just now and mixed a bit into water to display the color.
The compound is molybdenum blue, a hydroxide of molybdenum, Mo4O10(OH)2. It's a dark royal blue powder. The preparation was followed almost as per
Brauer, except I mixed the MoO3 powder into the dilute HCl very well, and then added the Zn granules:
"MOLYBDENUM BLUE, Mo4O10(OH)2
Obtained by reaction of nascent hydrogen with MoO3.
Fifty ml. of distilled water and 10 ml. of cone. HCl, followed
by 3 g. of analytically pure zinc granules, are added to 10 g. of
MoO3. The mixture is left standing overnight; the blue precipitate is then filtered off, washed until no chloride reaction is evident, and dried over
P3O5.
Alternate methods: a) Reduction with SnCl3 • 2 H3O in HC1
solution.
b) Synthesis from MoO3 and Mo powder (O. Glemser and G.
Lutz, see below).
PROPERTIES:
Formula weight 477.82. Blue crystalline powder. In air,
oxidizes very slowly to MoO3. Stable to NH3 and alkalies. Good
electrical conductivity.
REFERENCE:
O. Glemser and G. Lutz. Z. anorg. allg. Chem. 264, 17 (1951)."

[Edited on 13-2-2013 by Mailinmypocket]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by Xenon1898  |
Good use of a microscope in the lab. I am wondering, do many people find microscopes useful in the home lab? This seems like a great example of a
good use (two pure crystals forming as the same time). Seems like it would be a reasonable investment as an analytic qualitative tool to identify
crystal structure(?) |
That one wasn't actually taken under the scope, but I do have a USB microscope. I don't currently know enough about crystals to do much
identification, but I am sure that in the right hands it would be a powerful tool. Thusfar, I've mostly been getting up close and personal with dead
bugs, my warts/moles/hangnails, etc. Here's some of the crystal shots I took thought.

Copper acetate feathers

Closer view of copper acetate

Surface of a copper sulfate crystal

Thermite leftovers

Epsom salt
I'm really glad I got it - next I'm gonna get some polarizing filters and do some video of vitamin C 
edit: bugs etc can be found here
[Edited on 19-2-2013 by mayko]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
A tiny amount of Elephant Toothpaste that I had leftover after a large scale demo. Rather than washing it down the drain, I mixed the few drops of
each solution that was left over and it foamed up mightily  There's a bit of
starch added in to color the foam blue. There's a bit of
starch added in to color the foam blue.

|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

Long ago someone mentioned that how bad could be when something is overreacting... Someone in our lab had a large scale nitration (3 mol aromatic
aldehyde and a lot nitric and sulfuric acid) what was accidentally left over.... Do not try to reproduce it.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Crystals of copper(II) acetate:

|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
how do you make them grow like that? that's really cool!
all above information is intellectual property of Pyro.  |
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
That photo is taken from Wikipedia.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

Finally a successful nitration. This time bit more than 400g of 4MeO-acetophenone was nitrated
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Microwave Powered Steam Distillation
Out of the endwise vertical microwave comes a 29/32 to some addition thingie, to a splashhead and away through a 40cm high performance condensor to
the far far away collecting flask.
800 nominal Watt of MW power, when heated up it steams 4-500ml in 20 minutes on 80%, cleaner and faster then a steamgenerator.
And it looks beautiful as I recognized when sorting the pics today 
/ORG
[Edited on 24-2-2013 by Organikum]

|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Thats now only indirectly chemistry related but I think it is cute so I hope I will be forgiven.
And its a riddle: What is it?
 
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
It's a geared motor with a low rpm output shaft. Peristaltic pump?
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Copper ammonium oxalate. The crystals were so tiny that they had to be microphotographed, but I was quite pleased with them.

|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Guys, there's more and more great photos here, well done. 
Anders, how big are those awesome crystals? 5 mm?
Here's a really overexposed photo of you know what. 
(It was taken during the time I was purifying the accumulated batches. I don't work with it anymore. Thanks to my friend for letting me use his
camera.)
<img src="http://i135.photobucket.com/albums/q136/endimion17/pokusi/cuttingpurewhitephosphorusindark.png" width="800" />
I think it was a half minute exposure, perhaps more. I was really afraid I was "tickling the dragon's tail" as it looked as it was few seconds short
of bursting into flames, but had to be very still with the knife and the tweezers.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image width]
[Edited on 7/7/13 by bfesser]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
That's really amazing! It seems to glow like a light bulb. 
Here are some of my copper compounds: acetate and sulfate.
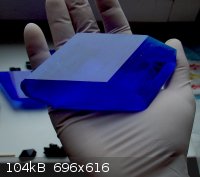
It now weights about 500 grams and that's enough. 

Some copper acetate crystals

The largest one...

...and a smaller one.
All of them were grown from only metallic copper, acid and air. The formation of these two salts from these 3 reagents took 1-2 years and the growth
of the crystals took 3-5 years.
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
That would be the turntable synchronous motor for microwave ovens.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Pok! Amazing copper crystals!!
On the subject of crystal growing, has anyone tried growing crystals in gel? Came across this page last week...
http://chemmovies.unl.edu/chemistry/smallscale/SS072.html
[Edited on 3-3-2013 by Mailinmypocket]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Hmmm, interesting... I haven't tried it myself, but I have crystallized CuSO4 with a low temperature procedure.
I wonder how many incredible Ru crystals would form if you melted ruthenium and kept the temperature just below its melting point, decreasing it each
day very little, letting it crystallize very slowly, forming enormous metallic crystals!
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Pok, those are spectacular. I think you could make some serious money if you sell them.
|
|
|
| Pages:
1
..
28
29
30
31
32
..
40 |