| Pages:
1
2
3
4 |
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Magpie
Acetone feed rate was 2mL/min, and acetone recovery was 38%.
I think the low flow rate was my main problem. Next time I will load the quartz tube with broken porcelain (per OrgSyn) or copper mesh (per NERV) to
improve heat transfer and reduce residence time in the furnace. Also I will increase the acetone feed rate hopefully to 4mL/min.
|
The following notes in the procedure from Org.Syn. may hold the answer to your results:
"4. With an electric combustion furnace, wherein a temperature of 695–705° is maintained, consistent yields of 35–40 per cent ketene are
produced. The best rate of flow in such a case is 4–6 cc. per minute, with recovery of 60–80 per cent of the original acetone as
distillate. Although yields of ketene ranging above 45 per cent have been obtained frequently with this apparatus, they could not be
duplicated consistently.
5. The thermal decomposition of ketene into carbon monoxide and ethylene is prevented, as far as possible, by the rapid removal of ketene
from the hot tube, which is accomplished by the undecomposed acetone vapor. About half the acetone originally used should be collected
unchanged as distillate by the vertical condenser. The yield of ketene will fall considerably if less distillate is formed.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
All this is in the literature.
"In order to obtain good yields of ketene from acetone it seems to be essential to decompose only a small fraction of the acetone. Factors such as the
temperature of decomposition (provided it is not much below 600°), time in the furnace and the presence of inert gases do not appear to have any very
appreciable effect on the yield of ketene except in so far as they affect the fraction of acetone decomposed.
Laboratory Preparation.
Ketene can be prepared conveniently by dropping acetone directly from a separatory funnel into a quartz [or Pyrex, but not iron - JACS 47, 1427
(1925)] reaction tube heated in the range 650-750°. The rate of introduction of the acetone is adjusted so that from 10-40% of the acetone is
decomposed."
JACS 56, 1760 (1934)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you Klute.
Run #3 was in progress today. Everything was going well, adding acetone at 4mL/min, when all of a sudden I saw no bubbles in the GAA
absorber/reactor. Checking all connections the cause was apparent: the polyethylene hose had sagged and was perforated just at the outlet of the
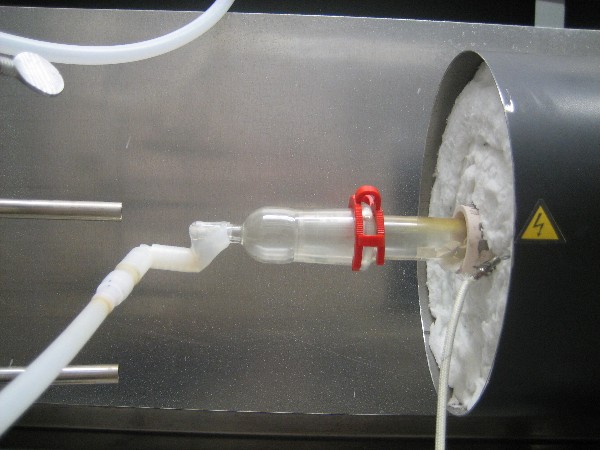
furnace. See picture below. I don't know if this was caused by ketene, or hot gases, or both. But the glass at that end never was hot to the touch.
When I saw that perforated PE tube I was very glad I had a good hood fan. I had completed about a third of my planned addition of 240mL acetone to
dose 80mL of GAA.
Also, I lost my acetone vaporizer due to massive cracking (luckily not breached, however). I had it really hot so I could be sure and get the acetone
vaporized immediately upon addition from the dropping funnel. I guess this is what happens when you violate the rule of "do not distill to dryness."
In that regard I was probably lucky that I aborted the run. I might have had an acetone fire otherwise.
I was happy the way the run was progressing and recovery of acetone was 61.3%. Now it is back to the drawing board.
I had the quartz tube loaded with broken porcelain per OrgSyn. I figured if nothing else this would cut down on residence time in the furnace.
Furnace temperature ranged from 689-711C. When the furnace cools off I'll post a picture of the tube to show any char.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's the picture of the quartz tube packed with broken porcelain from Run #3. As you can see there is virtually no char.
I gave some thought to what temperature would be best for the GAA ketene absorber/reactor. From an absorbtion standpoint an ice-bath might be best.
But from a reaction standpoint a high temperature, such as the 80C specified in the Chem E project posted by not_important in the acetic anhydride
thread, might be prefered. I suppose it depends on wheter the conversion to Ac2O is mass transfer limited or kinetics limited. Not having a strong
opinion either way I left the column at room temperature. Any thoughts?
I am pleased that I was able to achieve the OrgSyn feed flow and acetone parameters and no char. I haven't fractionated the product yet so don't yet
know whether I was successful overall. But I obviously have some equipment issues to resolve before I can continue.
[Edited on 26-9-2008 by Magpie]

|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
You might try a dry run with compressed air to see if it's just purely a temperature issue. Do note, however, that the heat capacities for nitrogen
and oxygen are different than that of acetone and ketene.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Watson that is a good suggestion. But I feel that I have to deal with it no matter the cause, and high temperature is most likely it. My plan is to
make a jumper out of glass tubing, butt the ends right up to the hose barbs on the adapters, and hold the joints together with heavy wall rubber
vacuum hose, as per OrgSyn. When all else fails I try following the procedure. 
What is your opinion on the best temperature for the GAA/ketene absorber/reactor?
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Magpie, earlier in the thread, NERV and I used an ice bath to cool our GAA, the gas was also bubbled through a frit, not just a plain tube. This
produces many small bubbles. It's in the pictures. It worked OK. I don't remember the final yield for the reaction, we got 60 some mL of Ac2O from 250
mL acetone.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks Fleaker. I have a fritted tube also but have been using just an open tube. It seemed like I was producing so much gas (in the first two runs)
that I didn't think I could use it. I'll give it a try. On run #3 most (61.3%) of the acetone was recovered so the gas production (ketene, methane,
ethene, etc) may actually be less voluminous. It did appear so as I recall.
Yes, for temperature of the GAA absorber/reactor, you were successful with an ice-bath. This is a strong argument for using same.
[Edited on 28-9-2008 by Magpie]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
How Control acetone flow rate in Quartz tube?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
With a pressure equalizing funnel. See photo upthread.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I did it one time and my rb flask break
First i heat my flask and then add acetone drop by drop
Flask temp was 100c
Now i control flow rate by temp of heater
I use 2lit flask,1meter 2mm(inside diameter)Quartz tube with Copper wire inside,50cm in furnace(temp 700c)
My ketene yield is little and my recover acetone is Yellow!
[Edited on 9-12-2009 by Waffen SS]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Initially I was heating a 500mL RBF with a mantle to serve as acetone vaporizer. My RBF cracked too - ouch! So I switched to hot water bath heating.
But this is a pain also. The best vaporizer I believe would use an oil bath with closed loop temperature control.
I have invested a lot of time and money into trying to get this ketene method to work. I never did get any Ac2O. It's also a very risky method. I
have now switched to S2Br2 for making Ac2O.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
But i got Anhydrid, now i am working for best perfomance.
If you like i can share my Result and Pic.
Also i am working on control flow rate part with new idea.
Final Product contail 3 componet after fraction distil:first come in ~100 that is soluble in water and has less density(i dont know what is
that).second acetic acid. third acetic anhydrid.
Your acetic acid and acetone should be dry for best result.
I believe best vaporizer is metal flask.
[Edited on 9-12-2009 by Waffen SS]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
What proof do you have that it is actually Ac2O? Try reacting it with aniline if you have any.
What is the yield?
The only significant variable that I have not yet tried is use of copper internals in the quartz tube. This was used by Fleaker/NERV in their
succesful run.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by Magpie  |
What proof do you have that it is actually Ac2O? Try reacting it with aniline if you have any.
What is the yield?
|
Proof:
-Boilng point:138c-141c
-React with water(produce acetic acid)
-Odor(strong acetic acid)
-If you add one drop anhydrid to water it will fall at bottom like oil and will disappear after a while.
Unfortunately i dont have aniline but i am sure that is anhydrid.
Now yield is little but i am improving it.
I used 1800cc acetone and recover 1500cc and got 30cc anhydrid.
Yield is little because most of ketene escape from absorber and i should use 2 or 3 absorber also i should contol
flow rate better.
Recover acetone is yellow and i guess ketene decompose cause that also there is unknown componet that has lower boiling poin(~100) that i dont know
what is that yet.
[Edited on 9-12-2009 by Waffen SS]
  
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Waffen, are you seriously attempting to make ketene outside of a fume hood? I can't tell for sure.
You say the ketene escapes the wash bottle. Does it by any chance go into the air that later enters your respiratory tract?
If so, I hope your health and life insurance is very, very good.
[Edited on 10-12-2009 by entropy51]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's an interesting looking tube furnace Waffen. Is it homemade?
I also managed to get a few mL boiling in the range 136-138C from my last ketene run. I didn't have any aniline at the time to test it. But I did do
some other tests shown in the link below, with mixed results. When I produced Ac2O with S2Br2 I knew I had relatively pure Ac2O right off the bat,
without even testing it. "A true huntsman knows a hunting dog, just as a true hunting dog knows a huntsman." or something like that. 
http://www.chemistry.ccsu.edu/glagovich/teaching/316/index.h...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by entropy51  | Waffen, are you seriously attempting to make ketene outside of a fume hood? I can't tell for sure.
You say the ketene escapes the wash bottle. Does it by any chance go into the air that later enters your respiratory tract?
If so, I hope your health and life insurance is very, very good.
[Edited on 10-12-2009 by entropy51] |
Yes Ketene is very toxic,i burn final mixed gas(ketene,methane,..)by burner and ketene in this temp decompose depend on your temp.
Furnace is self made and has digital display.
Now flow rate is low because little char produce in quartz.i am working on it.
Also i build complete system by copper metal but i have problem with that.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
| Quote: |
acetic anhydride is also prepared by the reaction of ketene with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar)
wikipedia
|
I think cold aceic acid will not produce good yield Ac2O.
We should heat washing bottle to 45-55c.
I got better yield When i heat my washing bottle to 50c.
Does ketene react with anhydrid acecic?
Ketene react with my PE tube and i think it absorb water from PE and remain char.
[Edited on 14-12-2009 by Waffen SS]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
You don't need a fume hood if you select scrubbers / washes properly. Use a wash, after the one for (partial) absorption, containing something it will
react with to a none harmful form; e.g. if something is exhausting acidic fumes, I'd wash it with a strong base. Wash flasks cost tens of dollars, can
actually neutralize exhausts as opposed to absorb them and can be used for other things.
I'm very impressed by all this furnace building and ketening. 
[Edited on 2-7-2010 by peach]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
We have not heard from Waffles SS for a while. Perhaps he has succumbed to his own ketene, which is very toxic (in spite of which the Nazi SS did not
think of using it as a poison gas during WW2).
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
That would be most unfortunate, as he was obviously quite capable given his construction of a most admirable tube furnace.
I would like to revisit this experiment on a larger preparative scale.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
I attach a small bubbler after the main one, and sometimes check it by samling it with a syringe and smell it, when it starts to smell acetic, and the
main bubbler heating ceases, I consider the glacial acetic acid => acetic anhydride conversion finished.
The cooling of the main bubbler is essential as ketene to acetic anhydride is very exotherm, If you do not cool it, acetic anhydride will vaporize,
and makes it to the second bubbler, so keep it as cool as possible, at mine desing even at -30°C the bubbler inner temp is about +50°C
When I checked the plastic tubing during operation one connection slipped apart, I suddenly breathed out and held back my breath while I fixed the
leak.. After a couple of seconds of the leak when the ketene reached my face, it was so intense and painfull like a punch.. especially my eyes burned
like hell, I cannot imagine what would ketene do to you after a lungfull of it, it must be very painfull death..
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Have you ever taken a good lungful of rich HCl(g)? How doest ketene comparuth?
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
HCl, Cl2 and croton aldehyde are nice ones too
Its like a sniff from glacial acetic acid but more permanent.
There are some chemicals which smell you had better not know, including ketene, Ac2O is also a nice one be contented with that
|
|
|
| Pages:
1
2
3
4 |