| Pages:
1
2
3
4
5 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Interesting. That is exactly what i saw when reading the stretch-o-meter : the force increased, reduced, then increased again while turning the handle
as regularly as possible.
Up near the snapping point the force would go a bit ape, increasing a Lot before reducing a lot just before the snap.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Up near the snapping point the force would go a bit ape, increasing a Lot before reducing a lot just before the snap. |
Which is why commercial dynamometers use a constant extension rate (200 mm/min, IIRW).
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
A quote from the website:
| Quote: | | Franklin and lots of other people have repaired their dishwashers with Sugru where it needs to withstand harsh detergents and high temperatures on a
daily basis. |

It'd be useful, I guess, but isn't there some sort of silicone dip that would work for most chem apps anyways?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The Volatile Chemist  | A quote from the website:
| Quote: | | Franklin and lots of other people have repaired their dishwashers with Sugru where it needs to withstand harsh detergents and high temperatures on a
daily basis. |

It'd be useful, I guess, but isn't there some sort of silicone dip that would work for most chem apps anyways? |
Assuming Franklin et al have been patching up any damaged EPDM based washing machine gaskets, I can assure you such a fix would be very temporary: Sugru is no match for highly developed, taylor-made EPDM
formulations.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The cured stuff does not like being submersed in conc sulphuric or nitric for a day or two, yet neither NaOH nor HCl seem to affect it :

The HNO3 treated sample has essentially retained it's original shape, although the colour has gone and the consistency is very much like
putty.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | The cured stuff does not like being submersed in conc sulphuric or nitric for a day or two, yet neither NaOH nor HCl seem to affect it :
The HNO3 treated sample has essentially retained it's original shape, although the colour has gone and the consistency is very much like
putty. |
Although a visual inspection is fine, usually loss of mechanical properties is used as a measure of how chemically resistant a material is. So, TS and
E@B after the treatment.
Try also immersing in a aliphatic solvent like kerosene. See if it absorbs it: weight gain (or loss).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What's an aliphatic solvent, apart from kerosene (which i don't have) ?
Is Aliphatic the opposite of Aromatic ?
[Edited on 14-6-2016 by aga]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Aliphatic refers to saturated alkanes (no double bonds, which would be olefinic). So, things like pentane, hexane, heptane, ligroin/petroleum ether,
naphtha, and so on. If none of those are available, there's always gasoline/petrol.
[Edited on 14-6-2016 by Crowfjord]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks Crowfjord, and glad to see you again !
Petrol is now my aliphatic solvent of choice.
I have some of that.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Petrol is not a good choice. Lighter fluid, like for zippo lighters, is a very aliphatic solvent.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Pretty sure that Lighter fluid is still available, although it's composition (here) is unknown.
I'll get some and find out.
Edit:
Actually no, i'll not, these guys will :

They got slapped on the glass, rolled a bit with a steel rod, trimmed to ~10mm width, left to cure for 5 hours then scraped off the fume hood glass
with a razor blade so they could cure freely overnight.
It took some acetone to get the remains off the glass - they're certainly sticky !
[Edited on 15-6-2016 by aga]
Oh dear.
I see that i just released an image of myself in the reflection, clearly showing the spok-like ears, oversized left eye and cranially-integrated
imaging prosthetic.
That's not a stripey t-shirt, it's my gills.
[Edited on 15-6-2016 by aga]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Lighter fluid is usually comprised of a strongly hydrotreated light naphtha, hence very aliphatic.
[Edited on 15-6-2016 by deltaH]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | | Also interesting: extend to say 50 % then stop extending and monitor stress. As a visco-elastic material its stress will reduce in time, aka
stress relaxation. |
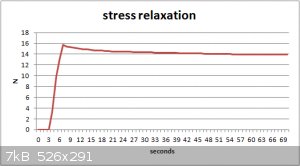
Lika dat ?
Stretched a small piece to 50% then held it there.
Had to video the scales then step through it to capture the readings.
Stretchy-snappied some more bits :
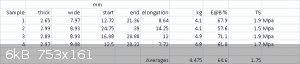
(WHOOPS : typo in the first image posted)
6 cured strips left.
What next ?
[Edited on 16-6-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
So that's a stress relaxation of about 12.5 %. Not bad, actually.
The other results still mystify me. The TS values are in agreement with Sugru's datasheet but the E@Bs fall well short (211 % advertised).
I wonder if the shape of the sample has something to do with that. Do the samples snap more or less at the middle (between the clamps) or at
either side where you clamp them up?
If the latter is the case, that's why 'pro' tests use the dumbbell shape: that way stress is highest in he narrow section and it then breaks somewhere
in that section.
Next, assuming you're up for it, clamp a sample at 50 % elongation for 24 h. Then release it and measure residual elongation 1 h after release.
Perhaps compare with another rubbery material, for reference?
[Edited on 16-6-2016 by blogfast25]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
SWAN brand (yellow can) lighter fluid, is distilled (says something like extra distilled and clean) on the tin. Also says its napha on the tin. I know
your not in UK but SWAN and brands like that with extra clean on the label should be napha and say so on them.
I used a couple of tins to try out as a extraction solvent, but gets pricey buying it like that if you need any volume.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Some did break where they are clamped and i also had some slip out of the clamps rather than break, so messed a bit with the clamping force.
The last entry on that table snapped exactly in the middle.
Does the thickness/width make any difference to the test ?
It seems like it should : thinner breaks sooner etc.
Edit:
A piece is now on the rack overnight, all stretchy-like.
[Edited on 16-6-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
The last entry on that table snapped exactly in the middle.
Does the thickness/width make any difference to the test ?
It seems like it should : thinner breaks sooner etc.
|
'On paper', thickness has no influence on E@B. The sample is supposed to break when stress exceeds TS. The stress is of course the force divided by
the cross section of the sample, which already accounts for the thickness.
For some projects we used to use different size dumb bells. The difference in observed E@B was always quite small.
Maybe the Sugru datasheet is telling porkies?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The 'rack' is currently occupied, so i can't test the other bits just yet.
If my results with a home-made stretchy-snappy-o-meter come out seriously lower than their claimed elasticity, it should be no problem to just say
that i did it wrong/the machine is crap/drunkards can't stretch/white men can't jump etc. etc.
That said, 65% E@B is a lot lower than 211%.
Nobody would care anyway.
Hmm. Perhaps the designers would.
Perhaps they could get their money back from the testing house that did it for them.
Calibration !
What are professional stretchy-snappy-o-meters calibrated with ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Nobody would care anyway.
Hmm. Perhaps the designers would.
Perhaps they could get their money back from the testing house that did it for them.
Calibration !
What are professional stretchy-snappy-o-meters calibrated with ? |
The load cells are calibrated with standard weights, the extensometer (that measures elongation) with a good quality ruler.
I think I need to buy some Sugru myself.  75 % E@B is a really
shockingly low value for what's being hyped/branded as a 'wonder material'... 75 % E@B is a really
shockingly low value for what's being hyped/branded as a 'wonder material'...
[Edited on 16-6-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It is possible that i just screwed it up, failed to properly tighten the screws on the machine i built, then failed to read a digital scale correctly,
failed to turn the knob with any precision, and their Test Lab had MUCH better knobbing and screwing capabilities.
I'll email them and ask if we're wrong.
Edit:
These things never turn out well, but needs must ...
[Edited on 16-6-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | It is possible that i just screwed it up, failed to properly tighten the screws on the machine i built, then failed to read a digital scale correctly,
failed to turn the knob with any precision, and their Test Lab had MUCH better knobbing and screwing capabilities.
|
No. I deem it unlikely, actually. Your machine may be a little ad hoc and simple but there's no reason to believe it would underestimate the E@B
values to such an extent (75 % compared to 211 % is a relative underestimate of 60 to 70 !!) Especially as the TS values seem in line with Sugru's
specs.
I would only advise one more simple test: with an improvised dumbbell, cut with scissors from a bit of Sugru cured sheet. This is to eliminate those
possible clamp effects.
General dumbell shape reminder (scale it to requirements):
http://www.ptli.com/testlopedia/subs/Tensile-rubber-sample.a...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Overnight test :-
Start length: 15.47mm
Initial Force applied: 27.9 N (50% of max stretch)
Duration: 20 hours
Length afterwards: 17.16mm
Difference: 1.69mm
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Overnight test :-
Start length: 15.47mm
Initial Force applied: 27.9 N (50% of max stretch)
Duration: 20 hours
Length afterwards: 17.16mm
Difference: 1.69mm |
A tension set of about 11 %, which is not bad at all.
The tension set is a qualitative measure of crosslink density. In plain English: a low value shows the sample has been properly cured (an
uncured sample would have a tension set of about 100 %)
This also means that poor curing can be more or less excluded as the cause of the low E@B values.
Does this Sugru stuff smell of anything when it's curing?
[Edited on 17-6-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Yes. It has a particular smell which is not altogether unpleasant, yet certainly has an effect on these sinuses if sniffed hard.
The smell is not familiar, so no name springs to mind.
The best i can describe it is like a mix of an acetone/carbon tet-like solvent and burnt metal vapours (e.g. angle grinder meets steel).
No ammonia smell at all.
Today i stumbled across ICl (as in iodine monochloride) which is said to be useful for cleaving C-Si bonds.
Maybe making a bit of that and seeing how it affects the sugru material would be interesting.
The E@B issue probably isn't down to sample shape anymore, although a couple more packs have been opened and formed into the dumbell shapes, then left
to cure overnight.
Edit:
As an afterthought one lump was balled up again and had a bit of damp pH paper stuffed into it to see if the pH of the solvent was measurable.
Came out the green of pH 8.
[Edited on 17-6-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
You're not getting any whiffs of vinegar? pH 8 would be in line with that.
Look forward to the dumbbells results.
Cleaving Si-C bonds? I don't think there are any in the backbone: silicone rubbers have a -O-Si-O-Si- backbone:
https://en.wikipedia.org/wiki/Silicone_rubber#Structure
|
|
|
| Pages:
1
2
3
4
5 |