| Pages:
1
2
3
4
5
..
20 |
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
CrO2Cl2 in CCl4 is another way of preventing oxidation of the aldehyde.
I wonder if CH2Cl2 could be used instead of CCl4.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Please theoretic
read Marvins and my posts in this thread regarding the difference between "dehydrogenation" and "oxidative dehydrogenation".
This might clear the confusion.
Of course a plain coppertube is a bad catalyst - very good is copper precipated on zink by cementation and oxidising this catalyst first, then
reducing it with hydrogen or ammonia to gain copper structural promoted by ZnO.
This is a highly active dehydrogenation catalyst in special for EtOH.
|
|
|
fritz
Harmless

Posts: 49
Registered: 29-11-2003
Member Is Offline
Mood: No Mood
|
|
Preparation of K-chromate.
First you have to obtain Cr(OH)3 -but this should be not too difficult! to a suspension of the Cr(OH)3 KOH is added until pH is at 9-11. to this you
add 30% Hydrogenperoxide (violent Oxygene generation) H2O2 is added in 10-20ml portions as the O2-generation decreases. (if pH drops add KOH to the
mixture) At the end of the oxidation the solution should be yellow and no traces of a green color should remain. The sln is boiled until all O2 is
escaped. if necessary it is filtrated. Finally the solution is concentrated by boiling until it´s deeply yellow. now the sln is filtrated hot in the
five times of it´s Volume of ETOH. After cooling about 30min in an ice-bath the ppte is filtrated off. The recrystallating step from EtOH may be
repeated. The resulting is Potassium-chromate. For the production of acetaldehyde you have to add a sulfuric acid solution of K-dichromate to EtOH. In
acid solution chromate forms dichromate. So the only thing you have to do is to increase the amount of sulfuric acid. (I don´t feel like calculating
the new ratio right now)
Permanganate in my opinion is bad. I think it would create acetic acid because its oxidizing potential is very high.
The Acetaldehyde which destills off is collected in diethylether. To this sln. gaseous ammonia is introduced and aldehydammonia is formed which could
be separated by filtration. this may also be a good possibility for storage of acetaldehyd. To obtain it from the aldehydammonia 25g of this stuff is
solved in 25ml water. A (cold) mixture of 30ml sulfuric acid and 40ml water is added and the aldehyde is distilled off.
|
|
|
chloric1
International Hazard
    
Posts: 1159
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
OYour on to something
friotz, I think your on to something there with the aldehyde-ammonia adduct. Would be good for storage. Also, the steps from your chromate sparked a
rather important issue. Recycling dangerous goods. If you have trivalent chromium as a by product then you can reoxidize it to reuse it again. At least here peroxide is sold at health food stores at 35% concentration so I
dont think it would be a major problem. Really, our hobby is under close scrutiniy and if at least some of us can show that we are responsible in
handling toxic polluting chemicals, them maybe we can regain the freedom to explore our hobby. At least here peroxide is sold at health food stores at 35% concentration so I
dont think it would be a major problem. Really, our hobby is under close scrutiniy and if at least some of us can show that we are responsible in
handling toxic polluting chemicals, them maybe we can regain the freedom to explore our hobby.
Fellow molecular manipulator
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
I was playing around with various chemical equations today during some free time I found, particularly decomposing calcium salts of carboxylic acids.
Normally you get aldehydes (HCHO with Ca(OOCH)<sub>2</sub> and ketones
(e.g. CH<sub>3</sub>COCH<sub>3</sub> with Ca(OOCCH<sub>3</sub> and ketones
(e.g. CH<sub>3</sub>COCH<sub>3</sub> with Ca(OOCCH<sub>3</sub> <sub>2</sub> <sub>2</sub> . Btw, I chose calcium
because CaCO<sub>3</sub> is so readily available to me in the form of... chalk! . Btw, I chose calcium
because CaCO<sub>3</sub> is so readily available to me in the form of... chalk!  Easy to crush up, and all. Easy to crush up, and all.
I then got into the "double salts" (correct phrase?), such as Ca(OOCH)(OOCCH<sub>3</sub> . If this could exist, even in small amounts where distillation would be required, one could theoretically make
acetaldehyde via: . If this could exist, even in small amounts where distillation would be required, one could theoretically make
acetaldehyde via:
CaCO<sub>3</sub> + HCOOH + CH<sub>3</sub>COOH <s> ></s>
Ca(OOCH)(OOCCH<sub>3</sub> + CO<sub>2</sub> +
H<sub>2</sub>O + CO<sub>2</sub> +
H<sub>2</sub>O
Ca(OOCH)(OOCCH<sub>3</sub> <sup><u> <font face=symbol>D</font> </u></sup><s>></s>
CaCO<sub>3</sub> + CH<sub>3</sub>CHO
<sup><u> <font face=symbol>D</font> </u></sup><s>></s>
CaCO<sub>3</sub> + CH<sub>3</sub>CHO
If you need HCOOH, you could always use oxalic acid (maybe from Zud cleaner) and glycerin by using <a
href="http://www.rhodium.ws/chemistry/formic.acid.html" target="_blank">this method</a>. <a
href="http://www.rhodium.ws/chemistry/grignard.formic.html" target="_blank">This</a> may also be of interest.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
re: getting pyruvic acid
| Quote: | When you boil pyruvic acid with diluted H2SO4 you get acetaldehyde boiling out of the reaction.
Pyruvic acid is easily prepared from sodium pyruvate available over the net (unsuspicious not so expensive) or at the healthstore (expensive).
Stochiometric amounts of HCl should do the trick I guess. |
According to The Old References, pyruvic acid itself is prepared by distilling tartaric acid with KHSO4 at 200-250 C. And potassium hydrogen tartrate
is easily available (though a bit expensive) from grocery stores. Find a place that sells in bulk; I can get it for about $13/kg. If bought in a
little spice bottle it's probably more like $50/kg. Now you don't need to order from the net or expensive health food stores at all!
Of course, this depends on how pure your acid must be... some old methods leave products that are difficult to purify, or else the yields are bad.
PGP Key and corresponding e-mail address
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
The copper pipe method sounds nice, looks I will be doing some welding this weekend. Why is acetaldehyde in such high demand? Besides making
pentaerythritol what uses does it have?
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
anubis
Banned
Posts: 4
Registered: 12-2-2004
Location: closer than u think...
Member Is Offline
Mood: No Mood
|
|
maybe
try also methylation of formaldehyde.
there should be a catalist metal but i dont know which.
bonding two ch its easy tho.
one way might be:
ch3cl + ch2o --ni---> ch3cho
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
my patience is wearing thin
Navarone, Navarone's Ghost, Anubis:
Your writing is sloppy, riddled with errors, and provides little of interest even when one ignores its technical blemishes.
I have valuable advice for you:
The next time you post anywhere other than Whimsy, provide enough details to show that you've already investigated the topic you are talking
about. The investigation could be an experiment that you have conducted, or that you have seen someone else conducted, or even something that you read
in a book or journal or on a reputable website.
Whatever you post about, you should make a respectable effort to spell words correctly and in their entirety, and to use such perfect punctuation and
grammar that no 7th grade teacher of English would hesitate to give you an "A."
If you don't post about a technical subject, and don't post in Whimsy, then your prose should be rich and beautiful in addition to
technically flawless. If Vladimir Nabokov's ghost isn't green with envy, you have failed.
I hate cluttering up perfectly good threads with this sort of off-topic discussion, so let me be extra-clear:
If you are incapable of following the above advice, kindly go home and eat bleach and die.
PGP Key and corresponding e-mail address
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
Acetaldehyde is attractive as a general purpose two carbon synthon. Although I think that the best route would involve simple catalytic
dehydrogenation, how about the following?
The problem with simple (electrolytic or chemical) oxidation of ethanol is that primary alcohols are wont to overoxidise. Oxidize boiling
isopropanol instead. Pass the vapors up a fractionating column packed with an MPV (Meerwein-Ponndorf-Verley) catalyst. Inject ethanol somewhere in the
middle of the column, and remove acetaldehyde from the top. If low boiling azeotropes could be avoided, you get the equivalent of high temperature
dehydrogenation without the high temperature.
Organikum: Could you give details and/or references for the ZnO promoted catalyst? What temperature does this stuff work at?
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Reference:
Anorganisch-chemisches Institut der Technischen Universität München
Hochselektive Katalysatoren
zur Gasphasendehydrierung
von Alkoholen
Markus Ludwig Gitter
Teiweiser Abdruck der von der Fakultät für Chemie der Technischen Universität
München zur Erlangung des akademischen Grades eines
Doktors der Naturwissenschaften
genehmigten Dissertation.
A.D. 2002
translated from the text:
Dehydrogenation of EtOH:
Coppercatalysts with ZnO, CoO and CrO3 as structural promotors. The reaction temperature is limited to 270°C - 330°C for to reach a selectivity
towards acetaldehyde of 95%.
Yields (per pass) are limited to 30% to 50% this way.
The hydrogen produced as byproduct is clean enough for use in catalytic hydrogenations.
The preparation of the catalyst is a standard procedure for precipitating metal catalysts onto a metal support - I think I got it from some patents.
It is tried and true.
(it cost me a damned long time to find out what "structural promotors" means)
If you precipitate copper on zinc you will see that the copper grows on the zinc in form of fractal "trees" providing a huge surface area
later in the dehydrogenation. Thats the whole trick of the "structural promotion" of zinc.
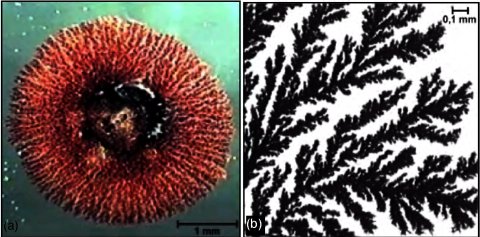
A coppertube filled with copper-scrubpads works fine if the amounts of acetaldhyde needed are not to large (less than one liter) - important is to
activate the copper by first oxidising it - blowing air through the tube at red dull heat - and reducing it again what can be conveniantly done by
passing ammonia or hydrogen through the hot tube.
It actually works also without this activation step. but yields are low.
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
A bit off topic; I apologize, but how did you know, Polverone, that anubis was Navarone? I saw the ammonium chloride thread where he was a complete
ass, but how did you recongnize him as opposed to some other idiot with bad spelling?
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
thunderfvck
Hazard to Others
  
Posts: 347
Registered: 30-1-2004
Location: noitacoL
Member Is Offline
Mood: No Mood
|
|
Check out the benzyl magnesium chloride thread, you will see. It's in general chemistry (reffering to anubis-navarone shitter).
[Edited on 17-2-2004 by thunderfvck]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I am good at recognizing writing-styles, at least where a single writer using multiple names does not make a good effort to alter styles between
names.
For example, if you search alt.engr.explosives on Usenet for "Rosco P. Coaltrain" or the E&W Forum for "Rosco Bodine," I think
you will have found our Mr. Anonymous. The writing styles are very similar and he discusses similar topics. I'm a little bit sad that after
finally dropping his cloak of super-anonymity he has not seen fit to post here at sciencemadness.
Oh, and I checked Anubis's signup IP address.
PGP Key and corresponding e-mail address
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Madscientist, you stated you managed to get 60 mL of liquid once you began to effectively cool the distillate. Can you describe how you arranged your
apparatus, was the copper just a straight or did you make a coil for greater heating area?
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
I gave the acetaldehyde synthesis a try today. I hooked up an apparatus which consisted of a flask connected to a heated copper coil connected to a
PVC condenser full of ice, connected to a flask. I started boiling about 550 mL of ehtanol and heating the pipe, and so far there is about 50 mL of
distillate, and it has a mild fruity smell, but I am not sure if it is mostly unreacted ethanol or acetaldehyde as they both have the same density. I
gave the synthesis a break for now because I noticed smoke coming back into the distilling flask. It was really weird it looked like when dry ice
sublimes, it was pretty dense smoke because it came back out of the hose and fell into the ethanol, I thought this might have been my acetaldehyde
going in the other direction, but I am not sure.
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
I also tried the heated copper method not so long ago. I used a 500ml boiling flask with about 1m of 3mm copper tubing. The tubing ran from the
stopper down to a coil around the bottom of the flask so that when on a gas cooker both the coil and the flask were being heated. The receiver was in
a bowl of ice.
I brought the ethanol to boil and collected the product which smelled mostly like ethanol with a slight fruity aroma or maybe that was just
imagination?!?
I see 3 main perameters that can be tweaked here:
1) The copper temperature.
2) The Ethanol temperature.
3) The length, size, and configuration of the copper tubing.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Plain copper tubing will not work well. Prepare a catalyst as described - it is easy like shit or at least use a tube filled with copper scrubbing
pads.
But hey - precipitating copper from the sulfateor better chloride is not rocket science.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by Polverone
I am good at recognizing writing-styles, at least where a single writer using multiple names does not make a good effort to alter styles between
names.
For example, if you search alt.engr.explosives on Usenet for "Rosco P. Coaltrain" or the E&W Forum for "Rosco Bodine," I think
you will have found our Mr. Anonymous. The writing styles are very similar and he discusses similar topics. I'm a little bit sad that after
finally dropping his cloak of super-anonymity he has not seen fit to post here at sciencemadness. |
Cheer up friend . Sorry for being cryptic , but it seemed prudent for some topics with folks like idefense and their echelon buddies lurking about .
Anyway , we all have some sort of alias on the web , or else my name is not Rosco P. Coaltrain 
My IP is as certain to be a fib too 
so I hope the logins here are not IP specific 
|
|
|
true_alchemy
Harmless

Posts: 21
Registered: 29-9-2004
Member Is Offline
Mood: No Mood
|
|
NaOCl (5%-bleach) in acetic acid, I believe, is a nice clean reaction for ketones from sec-alcohols. It's been 14 years since I have done it but
if I recall it works for aldehydes as well. ie ethanol added to clorox in acetic acid @ 20-30C. Clean up is done with sodium metabisulfite to kill
excess bleach. There are textbook procedures for this on the web.
[Edited on 1-10-2004 by true_alchemy]
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
I was just wondering about the method using H2O2 , EtOH, and a KMnO4 catalyist. Would you first add the KMnO4 to the EtOH, and then set of for an
addition reaction by putting your H2O2 into an addtion funnel. Also are you going to have the acetal, and ethonal by products that megalomania's
method has? Can you bubble ammonia gas through your crude product to get aldehyde-ammoina?
N/A
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Page 4686 of JCS (1956) claims a 50% yield by reflux of EtOH with excess MnO2 for 20 min. No explicit experimental is given in this case. A number
of aldehydes and a few ketones are prepared, but my copy (from microfilm) is of poor quality.
[Edited on 21-5-2005 by S.C. Wack]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
When I added 5ml ethanol to 50ml bleach (2,8% NaOCl), no chloroform was produced, but it still got warm and a strong fruity smell was being given off.
I'm sure that a considerable quantity of acetaldehyde had formed.
On addition of HCl, the smell got much more intense and the solution started boiling even though it was only mildly warm.
You know the boiling point of acetaldehyde?
I think this could be made into an economic method, because the precursors are so cheap and easy to get.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
The density of acetaldehyde is 0.783, the melting point - 123.4 deg C, and the boiling oint at 20.2 deg C. so volatile, although that is bound to be
different in aqueous solution.
I am not sure about this being a good route. I remember doing oxidation experiments with H2O2, and the smell was definitley there, but it's hard
to separate the aldehyde from the rest.
What I always wanted to do was to react acetylene with H2O in the presence of mercury salts, according to
HCCH + H2O --> CH3CHO
This is how it's done industrially. Shouldnt be impossible to do and the purity should be good, no ethanol contamination or anything else for
that matter.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Kalle anka
Harmless

Posts: 15
Registered: 10-6-2005
Location: Scandinavia
Member Is Offline
Mood: decomposing
|
|
Man! You guys must have written essentially all methods possible to make aliphatic aldehydes. But what comes to novel reactions here is something that
may be of some interest for you crazy people out there!
Now talking about acetaldehyde i want to tell you about an experiment of mine. One night i dreamt about several things but i woke up when it crossed
my mind. I wanted to make copper salts from metallic copper but as you guys know its reduction potential is higher than hydrogen, ie. acids wont bite
on it. A classical example in vitually all inorganic chems books i've seen is the demonstration of HNO3 as an oxidising acid. I have no access to
HNO3 nor H2SO4, so the reason i woke up suddenly was that i realised (omg im a n00b) that the NO3 ion is reduced.
My point is that i oxidised metallic copper which i took from some net cords and other cables with NaNO3 and HW grade 30% HCl. It needed some external
heating to start but then proceeded with a violent continuous exhaust of NO2 which settled as a reddish-brown toxic fog on the floor  Later i dreamed i wanted to see if i could do something else so i just grabbed what
we here in Sweden call "spolarvätska", dont have the english word for it but it is a solution of ethyl alcohol in water which is used in
the cars to rinse the main window. You could use any other available ethyl alcohol for this purpose. Later i dreamed i wanted to see if i could do something else so i just grabbed what
we here in Sweden call "spolarvätska", dont have the english word for it but it is a solution of ethyl alcohol in water which is used in
the cars to rinse the main window. You could use any other available ethyl alcohol for this purpose.
Anyway, as the relatively bearable chlorine like smell of NO2 could not have been enough, this shit made an awful pungent strong f*cking apple odour
even in my dream, which took a hell of a long time to exit. No appetite for apples 
As i made this in a ordinary 250ml rbf, all shit boiled off from the warm (~80*C) NaNO3+HCl solution and really just gave me a bad day. If i really
wanted to look at this possibility to make acetaldehyde, i would have set up a distillation apparatus and added 96% ethyl alcohol through a claisen
dropwise to the warm solution of HCl and NaNO3. I would also have liked to keep water through condensor cold and possibly placed the reciever in an
ice bath. But until then i think i just wake up and do something constructive.
Take care and have fun 
|
|
|
| Pages:
1
2
3
4
5
..
20 |