| Pages:
1
2
3
4 |
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by neptunium  | | i had a book long ago where the work of Marie Curie was detailled and the procedure had all the element extraction...for the life of me i cannot find
it ! |
The work you are referring to is either Marie Curie's thesis "Radioactive Substances" or her later book "The Discovery of Radium", both can be
downloaded from Archive.org.
Another level of interest in carrying out an ore separation process, modeled on Marie Curie's work is the essential importance of coprecipitation. It
was the isolation and purification of radium where the laws of coprecipitation were discovered and clarified. All of the people whose names are
attached to these laws (Fajans–Paneth–Hahn; Doerner-Hoskins) were radiochemists who worked with radium.
[Edited on 22-8-2014 by careysub]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i let sulfuric acid digest 70.3 g of monazite in order to start with the sulfuric liquor of thorium it is brownish yellow and is still disolving at
the moment...some chumks refuse to disolve though .
when finished (maybe another 24hours or so) i will filter it and run a spectrum on both filtra and solution...
should be interesting.
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
the filtra is pale yellow (the picture exagerate the color)

i believe most of the uranium and thorium should be in that solution.
could be wrong though but i pick up a lot more radiations from the precipitate than the solution.... a spectro gamma will surely clear that!
[Edited on 22-8-2014 by neptunium]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
this is the spectrum of the precipitate . wich seems to contain most of the activity... the sulfuric liquor may contain the Thorium and uranium
salt...
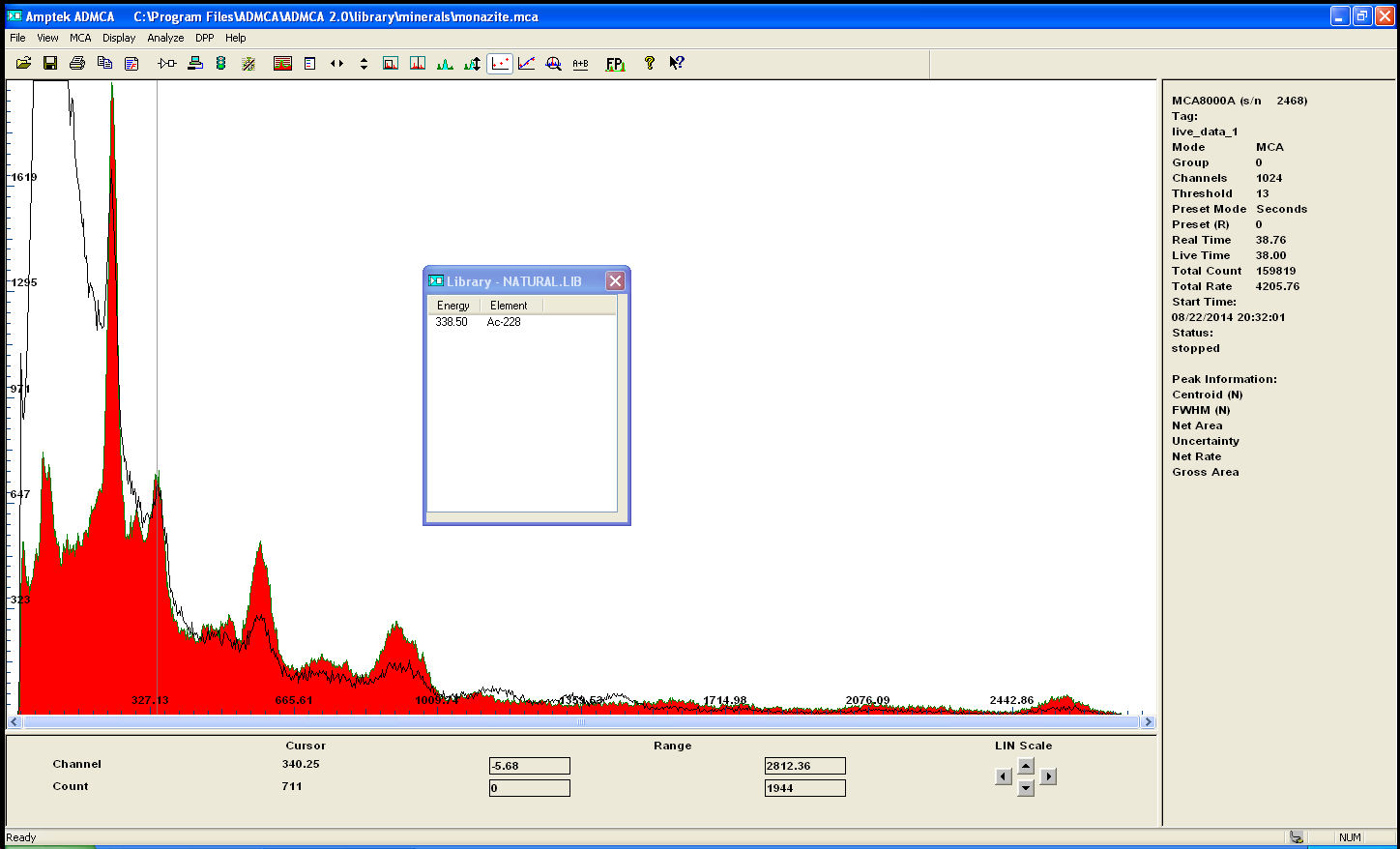
it isnt very suprising since lead radium etc sulfate will precipitate and stay in the filter..
i am going to disolve the rest in HCl and see what happens...what do you guys think?
[Edited on 23-8-2014 by neptunium]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
here is the precipitate from ammonia action on the filtra of the sulfuric liquor...
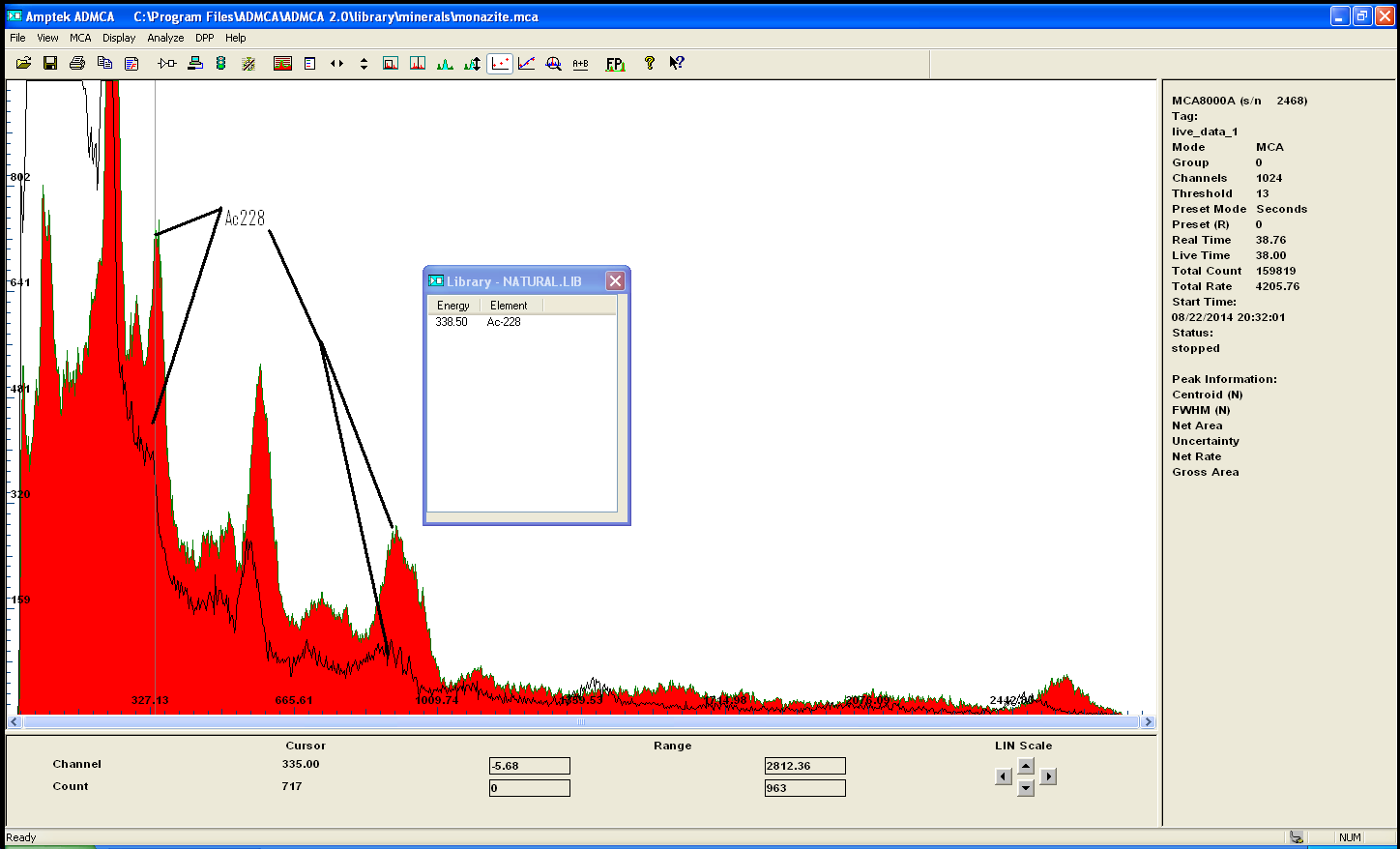
notice how Actinium stayed in solution but Th and lead at least are still there and even in higher amount the Thorium peak is off the chart and the Pb
hasnt changed very much...
let me remind you that this is 70.3 gram of ore to begin with and this precipitate is less than 3gram
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by neptunium  | the filtra is pale yellow (the picture exagerate the color)
i believe most of the uranium and thorium should be in that solution.
could be wrong though but i pick up a lot more radiations from the precipitate than the solution.... a spectro gamma will surely clear that!
[Edited on 22-8-2014 by neptunium] |
At last we have an actual ore extraction process post! Good show!
Where did you get the monazite?
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i have a few rocks sample from ebay....where else! lol
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by neptunium  | i had a book long ago where the work of Marie Curie was detailled and the procedure had all the element extraction...for the life of me i cannot find
it ! 
note that M Curie started with one metric ton of high quality ore and obtain 1 gram of radium giving its standart activity of 1 curie! ...
|
Marie Curie was really using uranium mining tails, the waste material after the uranium had been extracted. For her purposes this was better than
fresh ore since the mining company had done the first stage of concentration for her. The tails were 4.5 times more active than the ore, and being
waste at the time, it was free.
I am having trouble coming up with a specific figure of much tails she processed, "several tons" is the usual quoted amount.
There are hundreds of piles of uranium mining tails scattered over the uranium mining regions of the U.S. The NRC is charged with monitoring these,
and has a database of them - but I haven't come up a list yet.
On the other hand there is an accessible database of every uranium mine in the U.S. maintianed by the EPA: The Uranium Mine Database http://www.epa.gov/rpdweb00/docs/tenorm/402-r-05-009.pdf
You can download it as a DBF file (my OpenOffice spreadsheet program opens it as a spreadsheet without any problem):
http://www.epa.gov/radiation/docs/tenorm/uld-ii.dbf
The list is apparently very complete, when the Texas mines were compared to the state of Texas' own official list, it had every mine Texas knew about,
and some that it didn't. It includes also claims that were never actually mined.
Using Google Maps you can look up the geolocation of each of the mines for a little recon.
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
So you're thinking of doing a little midnight uranium hunting? Doesn't seem like the best idea, but I hope you succeed!
Edit: Wow, I had no idea that south Texas had so many uranium deposits!
[Edited on 8-24-2014 by zts16]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by zts16  | So you're thinking of doing a little midnight uranium hunting? Doesn't seem like the best idea, but I hope you succeed!
Edit: Wow, I had no idea that south Texas had so many uranium deposits!
[Edited on 8-24-2014 by zts16] |
The uranium minerals currently on the market are largely obtained by collectors frequenting old mine sites. People do this openly.
See for example:
http://carlwillis.wordpress.com/2008/02/20/uranium-chemistry...
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Out in the wilds zts16 you will likely not be bothered by anyone so long as you stay off of posted property. I have pounds of ore from many sites all
over Arizona which were long ago abandoned. Of course my collecting was done decades ago but I imagine abandoned locations abound to this day. Wear a
sidearm and watch out for Western Diamondbacks among other critters. IIRC John Wayne once said of the desert "everything wants to stick you stab you
or kill you".
Or in other words pay attention when collecting rocks around cracks and crevices where snakes like to hide from the heat. Better yet do most your
collecting during the winter months as I used to do. Wear gloves, don't breathe dust from tailing piles, etc., the usual common sense precautions.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by IrC  | Out in the wilds zts16 you will likely not be bothered by anyone so long as you stay off of posted property....
|
Or more affirmatively - the ticket is to stay on BLM land, unless some special restriction is in place, everyone is has a right to go there.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by careysub  | ...
I'm not sure you can find anything but low grades of uranium ore on the surface in the U.S. are any more. Uranium is still heavily mined, and the "low
hanging fruit" was dug up long ago. Surface deposits were prospected for very intensely during the 1950s and early 1960s.
If all you can find is 0.1% grade, then even at $10/g your ore would only be worth $10/kg and you have to dig up several hundred kg to finance your
prospecting expeditions just to break even.. |
I see the errors in my thinking now.
To the mining industry only bodies of minerals in quantities large enough to commercially mine are "ore", and must be extractable by (virtually)
labor-free mechanized equipment. This means they completely ignore small deposits of uranium, no matter how concentrated they aren't considered "ore".
And even after an area is "mined out" there can be a lot of hot rocks lying around.
Unlike a rockhound, miners aren't going to be scouting the ground with detectors.
So while 1% ore is considered "high grade" these days, there can still be much accessible material with content of 10% or even higher for the picking.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Marie Curie's Extraction
I tracked down Marie Curie's account of how much material she processed and how much radium she extracted.
She worked with 7 tonnes of tails, which was 4.5 times as radioactive as the original ore, so it was the equivalent of about 30 tonnes of pitchblende.
She isolated 0.2-0.3 g of radium, which is about 250,000 microCuries, and would exist in equilibrium with some 750 kg of natural uranium. This
indicates the original ore averaged 2.5% U.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
This is some wonderful chemistry! In future years I hope to go back to it, and repeat some of it.
| Quote: | | a chemical "script kiddie". |
Wow. That is SUPER accurate. Being a 'kiddie programmer' at a younger time, that hit home even more than the concept o cookery instead of chemistry,
or the kewls. Maybe a Quiche Eater Chemist (If you've read the letter 'Real Programmers Don't Use Pascal').
Regardless, it seems to me this would make a great subforum. Cost IS the major limit, but could be overcome. What would be problematic with larger
quantities of ore (for getting reasonable product) is reagents to reduce the ore to compounds workable.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
careysub,
When were you going to tell us what you were proposing wasn't legal under the regulations you were quoting?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Marvin  | careysub,
When were you going to tell us what you were proposing wasn't legal under the regulations you were quoting? |
Come on Marvin, if you want to debate this issue then do it.
State you what's on your mind, don't resort to a fake question.
You are awfully active on the Energetic Materials site, much of which directly violates criminal law, to adopt this faux offended pose.
If you want a debate you will have to uphold your end of the discussion.
Ante up.
[Edited on 4-9-2014 by careysub]
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Moved
11-9-2014 at 19:13 |
Polverone
Now celebrating 21 years of madness
|
Thread Topped
11-9-2014 at 19:22 |
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Wow, I just now noticed the new radiochemistry subforum... awesome!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's great! Congrats guys, making history on SM 
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
unbibium-292
have you all heard/read about the possible discovery of a super actinide
Possible natural occurrence
"On April 24, 2008, a group led by Amnon Marinov at the Hebrew University of Jerusalem claimed to have found single atoms of unbibium-292 in naturally
occurring thorium deposits at an abundance of between 10−11 and 10−12, relative to thorium.[29] The claim of Marinov et al. was criticized by a
part of the scientific community, and Marinov says he has submitted the article to the journals Nature and Nature Physics but both turned it down
without sending it for peer review.[30] The unbibium-292 atoms were claimed to be superdeformed or hyperdeformed isomers, with a half-life of at least
100 million years.[27]
A criticism of the technique, previously used in purportedly identifying lighter thorium isotopes by mass spectrometry,[31] was published in Physical
Review C in 2008.[32] A rebuttal by the Marinov group was published in Physical Review C after the published comment.[33]
A repeat of the thorium-experiment using the superior method of Accelerator Mass Spectrometry (AMS) failed to confirm the results, despite a 100-fold
better sensitivity.[34] This result throws considerable doubt on the results of the Marinov collaboration with regards to their claims of long-lived
isotopes of thorium,[31] roentgenium[35] and unbibium.[29] It is still possible that traces of unbibium might only exist in some thorium samples,
although this is unlikely.[27]
It was suggested in 1976 that primordial superheavy elements (mainly livermorium, unbiquadium, unbihexium, and unbiseptium) could be a cause of
unexplained radiation damage in minerals. This prompted many researchers to search for it in nature from 1976 to 1983. Some claimed that they had
detected alpha particles with the right energies to cause the damage observed, supporting the presence of unbihexium, while some claimed that no
unbihexium had been detected. However, the possible extent of primordial unbihexium on Earth is uncertain; it might now only exist in traces, or could
even have completely decayed by now after having caused the radiation damage long ago.[18]"
from http://en.wikipedia.org/wiki/Extended_periodic_table
it reminds me of the story of the curies. processing tons of ore.
i don't know much about this and am guessing that even though "A repeat of the thorium-experiment using the superior method of Accelerator Mass
Spectrometry (AMS) failed to confirm the results, despite a 100-fold better sensitivity".it may be possible that using a different technique to verify
or disprove the finding is not entirely scientific. and perhaps using the same teqnique, Replication, has a different outcome. it is not even
mentioned if they used the same ore.
[Edited on 22-11-2014 by quantumcorespacealchemyst]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
the island of stability has long been speculated to lye somewhere arround z=126 in the periodic table.
this claim if bold and has little if any proof. this Marinov guy has a lot of balls...
I wonder howany one could explain the formation of deformed nucleus in nature with an atomic number of 122!
supernovae do create element beyond iron but how far beyond the actinides? and how much of it would be left today when the last speculated closest
supernovae happened 5 billion years ago ?
even with a half life of 100 million years assuming traces amount at creation (considering how much actinides are left in nature today) thats still 50
times its half life! not much is usually left after 10 periods...
sounds bogus.
[Edited on 6-12-2014 by neptunium]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by neptunium  | ...
even with a half life of 100 million years assuming traces amount at creation (considering how much actinides are left in nature today) thats still 50
times its half life! not much is usually left after 10 periods...
sounds bogus.
... |
There was a claim in 1971* that Pu-244, with a half-life of 70 million years (making it the longest lived plutonium isotope) had been detected in
bastnasite at a very low level, which would have made the shortest half-life nuclide of primordial origin to be detected on Earth. Recent studies
using material from the same mine, but using the far more sensitive accelerator mass spectrometry failed to find any at levels much lower than
originally reported, thus disproving the original paper.
However, it is really believed highly likely that there is some Pu-244 on Earth, but it may be so little that we will never detect it.
The is one primordial nuclide, Sm-146, with a half-life of 107 million years that has been detected with some confidence. But here we have the
advantage of being able to obtain large amounts of pure samarium from nature in which to look.
So *IF* there are super-stable super-heavy nuclides with half-lifes of 100 million years, then some should be here, and there is some possibility
that some atoms can be found.
* D. C. Hoffman, F. O. Lawrence, J. L. Mewherter, F. M. Rourke: "Detection of Plutonium-244 in Nature", in: Nature 1971, 234, 132–134
[Edited on 6-12-2014 by careysub]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
some of these element could have been formed by neutron capture from cosmic ray or spontanious fission.
lf a few atoms are distributed here and there the only way to tell if they are primordial or not would be in the evironment in which they are found.
working in the radio chemistry lab of a nuclear plant years ago ,i remember getting flags of Be7 on the spectrometer from time to time (53 days half
life) which is a part of a natural cycle . Although different situation,
exotic nucleides are present on earth from natural processes,
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Not Pu-244, or any superheavy element.
Creating these requires successive neutron captures in a short period of time, since some (or many) of the intermediate nuclides decay quickly, and
thus very intense neutron fluxes are required. In nature only supernova nucleosynthesis provides this, where it is called the r-process (for "rapid").
In non-saturation conditions (i.e. everywhere except supernovas or thermonuclear weapons) the production rate decreases exponentially with each
successive capture, so it drops to effective zero after a small number. Nature can make Pu-239, Pu-240 no doubt, maybe detectable amounts of 241 or
242 (not sure whether these have been detected) but that's it.
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
the natural reactor in Africa is the proof of what nature can do alone.. so if Pu244 cannot be generated, where does that leaves us for Unbibium 292
and Marinov`s claim?
exactly!!
|
|
|
| Pages:
1
2
3
4 |