| Pages:
1
2
3
4 |
MephistosMinion
Harmless

Posts: 24
Registered: 16-1-2005
Member Is Offline
|
|
Hardy har har... *shakes head*
I am now back in my beautiful home state of Tasmania after my overseas stint in Europe. I went and got some guar gum today and have -325 mesh Al set
aside for this experiment. The only thing I need is the 50% H2O2. I have a little 35% left but I imagine it will not suffice. I found a store that
carries it (albeit a little steep at $20 AU per 500ml)
I have moved my entire setup out to our property (90 acres of land) although I imagine I will not be able to pass this one a "rocket cato" to the
neighbors 
EDIT: Minor capitalisaiton and Americanistaion errors.
[Edited on 1-2-2006 by MephistosMinion]
|
|
|
hinz
Hazard to Others
  
Posts: 200
Registered: 29-10-2004
Member Is Offline
Mood: No Mood
|
|
About urea peroxide:
Has anyone compared the structure of acetone and urea? I'm wondering if you can't make an urea based explosive with a structure similar to acetone
peroxide, since both have a carbonyl group. This means that it might be possible that you peroxide urea via electrophilic addition of a H+ion to the
carbonyl double bound and later add (nucleophilic) a peroxyde cation (O-O-H)- to the C-atom to which the charge of the H+ has moved. Because the NH2
groups are more electronegative, they won't steal the possitive charge of the C+ ion. The only problem I see here that urea is a base and much more
acid has to be used than with acetone peroxide.
Maybe there is one hitch I haven't seen
In the attachment is the AP reaction mechanism from frogfoot, I hope I may post it here since it's deleted.
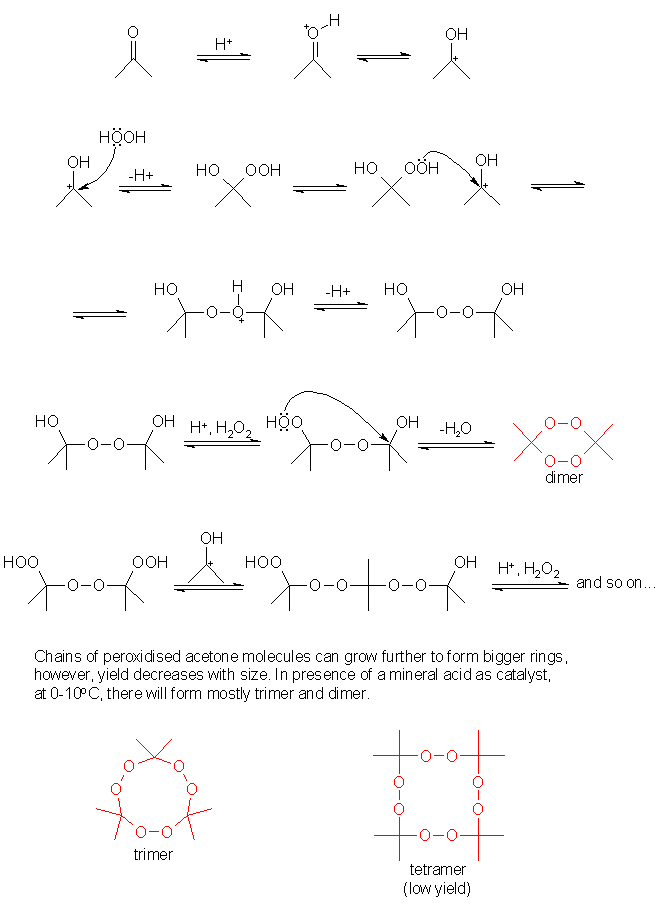
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
As to crosslinking compounds, there are numerous possibilities - and have potential for experimentation:
Acrylamide forms nice gels at 10% concentrations, in H2O2. Crosslinking, aka polymerisation, is achieved by ammonium persulphate (and TEMED,
N,N,N',N'-Tetramethylethylenediamine, but I am sure substitutes can be employed). With 50% H2O2, this should make an interesting polymer.
Others are of course agarose (did I mention this above?), which can be dissolved in the H2O2, and heated to ~60 deg C, to then let it set.
Another one is alginate, which is easily obtained from arts-retailers. From personal experience, it sets with the smallest amount of water.
Then, another possibility is to make bakelite-type gels/polymers. For this one needs formaldehyde, water/H2O2 and phenol. Alternatively, water/H2O2,
formaldehyde and urea also forms solid polymers. The same holds for guanidine base, CH2O and water.
These however may form primary explosives interspersed in the gel - so they could be quite interesting.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
"Binex 400"
Axt's mention of an explosive comprising water and powdered aluminum got me looking into it a little bit (the thought that it may be possible to make
such a potentially highly energetic material from two simple substances is fascinating, IMO).
Anyway, although I didn't find any examples of a commercial explosive based strictly on H2O + Al, I found something else I never heard of before:
Apparently there is a fairly "powerful" commercial explosive called "Binex 400" comprised of H2O, Al, and some sodium perchlorate (although the
proportions I don't know).
In case anyone is interested, I'll try to attach the paper I found regarding tests comparing "Binex" to some other high explosives.
I think I found a patent related to "Binex". US #5226986 gives examples of NaClO4, H2O and Al, but the Al in the examples is apparently not powdered
but granular, and not unexpectedly the mixture seems to be relatively insensitive, has a relatively large critical diameter and a low VOD comparable
to ANFO.
[Edited on 23-2-2006 by jpsmith123]
Attachment: Rickman_Demo_NDIA.pdf (497kB)
This file has been downloaded 1659 times
|
|
|
ZoSo357
Harmless

Posts: 16
Registered: 30-8-2005
Location: Here
Member Is Offline
Mood: I dropped it 
|
|
I've recently aquired a few pounds of 625 mesh Al, and am planning to test out some of this peroxide watergel. I have 35% H2O2 which I plan to test it
with. I'm thinking that I'll go about steaming some of the H2O2 up to a 50% concentration, but I also want to test out the 35% concentration since it
seems that nobody has tried this yet.
My problem is that I don't know how to properly adjust the ratio's for aluminum/H2O2 from 50% concentration to 35% concentration. Any help would be
greatly appreciated.
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by ZoSo357
My problem is that I don't know how to properly adjust the ratio's for aluminum/H2O2 from 50% concentration to 35% concentration. Any help would be
greatly appreciated. |
Dont change the ratios, theres no significant difference between reducing H2O or H2O2 to H2. There is going to be <1% difference between the ratios
when changing 50% to 35%.
|
|
|
ZoSo357
Harmless

Posts: 16
Registered: 30-8-2005
Location: Here
Member Is Offline
Mood: I dropped it 
|
|
I guess I'll stick with the 50:50 ratio then?
Now I basically just have to get guar gum or xanthan. If i become impatient before I get the chance to get out, I'll probably try starch, and note
it's performance. Thanks for the help.
EDIT: Also, how sensitive is this to detonation? I see you use .5 grams of PETN Axt (like with most of your dets) but I usually use Acetone peroxide
for detonators. Would a 1 gram AP detonator suffice for this?
Edit 2: I've mixed up a small sample of this to examine how it reacts. I added 2.5 grams of aluminum powder, 2.5 grams of H2O2 and 0.1 gram of starch.
it mixed into a kind of thick mixture somewhere between jam, and paint. It seems to have grown in mass so I would believe that would be from the
formation of air bubbles. Next step would be to try this again with a larger batch and see if it detonates.
[Edited on 22-6-2006 by ZoSo357]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Everything you always wanted to know about hydrogem peroxide
http://www.h2o2.com
http://www.h2o2.com/intro/properties.html
|
|
|
Marsh
Hazard to Self
 
Posts: 79
Registered: 28-7-2006
Member Is Offline
Mood: No Mood
|
|
(Sorry to have to raise this question as it has been briefly discussed in a few places, but I haven't exactly found a source...hope it is ok to ask in
this thread.)
If you don't mind sharing supplier info, where are you guys finding your H2O2 with over 60% concentration levels? I would distill the H2O2, but I am
rather limited with my current lab inventory.
I have so far managed to find one supplier for 60% H2O2, but this seems to be the peak of what my searching can bring from the net.
I noticed it has been mentioned about 70% industrial grade peroxide being readily available, but where might I ask is it so readily available?
In another thread, there is mention about the Baquacil Ultra spa cleaner with vented cap (I believe regular Baquacil is around 30%). Our local pool
supply carries this product, but at $34/500ml it is a bit hefty of a price. I imagine it will not top 55-60% concentration either, and if it was say
70% the price might be reasonable for me to use for testing. Has anyone yet gathered info on the Ultra's actual concentration level? (I'm really as
concerned though about Baquasil specifically either way, just curious for fact, as the price tends to keep me away.)
I would like to perform some experiments on the peroxide watergel explosives shortly, and continue the data. There seems to be some contradicting
(well, widely varied) info gathered, even from this thread, and I find it interesting to see what a wide span there seems to be. From what I have
gathered, it seems 50%+ H2O2 conc is desireable for sensitivity reasons, and 70% would(may) appear rather optimal in this scenario provided it is
easily accessed/available (although, I would like to test this idea by obtaining 70% concentration myself)...even though complete detonation occured
in the scenarios using 50% h2o2 w/ 0.5g PETN det...there still seems to be conflicting info when aluminum type used comes into play.
It seems to me that the aluminum used may be the number one contributing factor, once sensitivity can be satisfied by use of at least 50% H2O2 (maybe
even less concentration as stated, though). I do have both -600 mesh indian blackhead Al as well as -325 (possibly -400, need to identify under the
microscope) mesh standard Al powder. I would like to first be sure the latter Al is uncoated and thus non-painting grade, I do not believe it is
coated, but any ideas on finding this out?
I would like to attempt sensitivity tests between the various Al types, maybe even obtain finer Al than what I have, if it seems to be leading to
something. I would like to attempt specific sensitivity tests between various h2o2 concentration levels used also, provided I can aquire the higher
concentrations (60%+). 27% conc h2o2 watergel would be preferable, if it could be proven to be more easily detonated than what has been experienced.
Like Axt said, it may have only been 20% conc levels he was working with instead of the predicted 30% in the failed test.
I have a coal bucket which has 1/4" steel walls as well as 1/2" walls in various sections. I would like to dismantle this eyesore sometime and I
wouldn't mind doing it while testing the watergels.
The earlier photo of the watergel vs ANNM looks promising, even though it was less confined by its (plates) placement. I would like to test against
ANNM+Xylol/H2SO4 as well, but I have no NM currently so this may have to wait for some time. Afterall, these two explosives really haven't been
directly compaired, from what I can find.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You don't to distill the H2O2, as such. Instead you boil off the water, leaving the H2O2 behind. Use reduced pressure and a temperature of 35 to 50
C. This boils off a dilute solution of H2O2 that reaches 10% to 15% as the liquid reaches 70% H2O2.
Arrr - can't give you a reference, check 19th and early 20th century inorganic books for H2O2 preparation, they were always concentrating the dilute
stuff they got from BaO2.
|
|
|
Marsh
Hazard to Self
 
Posts: 79
Registered: 28-7-2006
Member Is Offline
Mood: No Mood
|
|
Thank you...for some reason I thought its bp was lower than water...stupid mistake I guess.
I would gladly purchase 70% if I can find it, to save just a bit more time.
If anyone could send me in the right direction, it would be really appreciated.
I have remained searching all morning, the 60% supplier I have found apparently has shut down and I cannot order. All I am finding is these darn MSDS
sheets all over the net for H2O2, yet no links to their suppliers when searching.
[Edited on 1-8-2006 by Marsh]
|
|
|
Marsh
Hazard to Self
 
Posts: 79
Registered: 28-7-2006
Member Is Offline
Mood: No Mood
|
|
Alright, I picked up 27.5% H2O2 from a pool supply, to be concentrated hopefully around 60-70%.
After leaving, I read that there is (I assume mild) concentrations of surface agents, whatever these are specifically for I do not know.
I realize these should be ok in this instance, but has anyone had problems with additives in conjunction with an Al mix or acetone peroxide? Sorry I
am getting off topic here but I figured there is enough talk about peroxide here to be somewhat relative.
If these surface agents might be a problem, I can return for food grade 35% peroxide from another supplier.
[Edited on 2-8-2006 by Marsh]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Most additives for H2O2 are stablizers, chelating agents and the like. That h2o2.com mentions the more common ones and the types of peroxide they are
likely to be used with (type as 'the intended application'). Surface agents sounds like the intended use is for cleaning or sterilizing surfaces,
where you want to make sure they get wetted by what you apply.
The phosphate based stablisers might cause problems with Al, as from what has been written here the Al must react a bit with the H2O2 to form bubbles.
If that turned out to be a problem, a small amount of alum solution could be added to the H2O2 just before mixing everything else up, as that would
react with the phosphates first.
As for surface agents, hard to tell. Would depend on which surface agents are used, and even then the concentration of them might make a difference.
|
|
|
ZoSo357
Harmless

Posts: 16
Registered: 30-8-2005
Location: Here
Member Is Offline
Mood: I dropped it 
|
|
I hate to bring up an old thread, but here goes.
I was looking into substitutes for guar gum, and i came accross something called "pectin crystals" which is a crystallized powdery substance which
comes naturally from certain fruits like lemons and apples. It's used in jams and jellies as a thickener. I plan on testing a mixture of 625 mesh
aluminum, "certo" brand pectin crystals, and 35% H2O2 some time this week.
Any thoughts on what this may perform like?
|
|
|
pyrotekniker
Harmless

Posts: 8
Registered: 3-12-2006
Member Is Offline
Mood: No Mood
|
|
I can easily get < 50 µm Aluminium powder. It is atomized, but is that good enought? Or do I need flakes?
In my garage, I allready got a some litres of 50% H2O2, which I'm going to try to make Watergel Peroxide out of 
[Edited on 9-12-2006 by pyrotekniker]
|
|
|
ZoSo357
Harmless

Posts: 16
Registered: 30-8-2005
Location: Here
Member Is Offline
Mood: I dropped it 
|
|
I know axt used paint grade aluminum (200 mesh) but im not totally sure if it's atomized or not. It may be since spherical aluminum gives more of a
shiny appearance than flake aluminum.
You could always try mixing a small batch and see if it forms bubbles.
EDIT: You can pretty well scratch any chance of the pectin crystals working. I tried mixing it in the proper ratio, and 2 or 4 percent pectin to
peroxide/fuel isn't enough to gel anything really.
[Edited on 9-12-2006 by ZoSo357]
|
|
|
pyrotekniker
Harmless

Posts: 8
Registered: 3-12-2006
Member Is Offline
Mood: No Mood
|
|
OK, guess I'm going to try a small batch then. Maybe I'm going to try to make gel out of it in some other way. Polyisobutylene maybe.
It seems like it is quite sensitive, going to try use a small comercial blasting cap.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
PIB isn't water soluable. Guar gum is easy to find and cheap.
http://www.amazon.com/Barry-Farm-Guar-Gum-oz/dp/B00015UC5M
|
|
|
Nixie
Hazard to Others
  
Posts: 490
Registered: 12-12-2006
Member Is Offline
Mood: ?
|
|
| Quote: | Originally posted by fatkanga
Dissolve urea in 2:3 molar excess of hot (60°C) 30% H2O2, then cool. CO(NH2)2.H2O2 should start crystalising out. then evaporate off
|
How long would I have to keep this on my teeth for whitening? Are the dentist whiteners based on carbamide peroxide mixed with something else? I'm
reading "pH optimized for whitening"... WTF does this mean, should it be basic or acidic? And how do you prevent burning your gums? Anyone know how
long the dentist-office strength product is kept on the teeth?
BTW what's a recipe for a suitable gel to dissolve this in so I can apply to my teeth?
[Edited on 9-2-2007 by Nixie]
\"Good is a product of the ethical and spiritual artistry of individuals; it cannot be mass-produced.\" --Aldous Huxley
|
|
|
E-tech
Harmless

Posts: 36
Registered: 30-5-2007
Member Is Offline
Mood: No Mood
|
|
Old thread, but interesting, to say the least.
Found that a mixture of 42% Al powder, 30.13% AN, and 27.87% water made a cap-sensative explosive with a 4000M/sec detonation rate.
The interesting thing about the mix is that it the edges of the metal witness plate it was tested on were partially melted after the detonation.
Check patent #2,836,484 "Aqueous metal powder explosive" for details.
interestingly enough, the military has re-discovered the fact that combustion of Al in the air is more effective in a confined space than most
explosives.
do a google search for "Shock dispersed fuels" or "Combustion of shock dispersed flake aluminum- high speed visualization"
Thermobarics fans will love it.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Now replace the 28% H2O with high percentage hydrogen peroxide and see what happens. 
|
|
|
E-tech
Harmless

Posts: 36
Registered: 30-5-2007
Member Is Offline
Mood: No Mood
|
|
just what I was thinking- even a weak 3% peroxide should help, but higher strength would likely be a lot more fun!
Very glad I got that rock tumbler for Al powder making- I'm gonna need to keep it running!
by the way, 1.5 grams of hmtd or tatp is equal to a number 8 blasting cap- capable of setting off detcord and civilian explosives.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by E-tech
Old thread, but interesting, to say the least.
Found that a mixture of 42% Al powder, 30.13% AN, and 27.87% water
made a cap-sensitive explosive with a 4000M/sec detonation rate. |
Very funny , was that actually measured out to two significant places ?
Post this expedient method to measure VOD - that is far more interesting.
Varying the ingredient composition only alters the detonation sensitivity
which for this type of composition is already at the high end of insensitivity.
The singular desirable property of this mix is the highest available energy
per unit weight, and that expended only as blast rather than brisance.
H2O + Al alone ( If it could detonate ) would yield 1760 Kcal / Kg
NH4NO3 + Al alone yields ~ 2400 Kcal / Kg
You won't improve on the military formulation which yields 2350 Kcal / Kg
Al 40 % , AN 40 % , 20 % H2O + polystyrene soap binder
http://en.wikipedia.org/wiki/BLU-82
VOD is well under 5 - Km/ sec , and requires IIRC some 16 or so fuzes
girdling the base to set off reliably because without suitable confinement
the slurry tends to be dispersed by the explosion rather than propagating
detonation, lowering the intensity of the blast wave in air.
More interesting are solutions at some concentration of H2O2
for example : 3 parts 90 % H2O2 solvates 2 parts NH4NO3.
Very pure H2O2 or its concentrated water solutions cannot be made to
propagate detonation except confined at critical diameter, initiated by a
booster. R-OH or unsaturated hydrocarbons present at some percentage
will render it highly shock sensitive, VOD well over 7 Km/ sec.
In this respect it is no different than water. Sensitizing without
inducing rapid decomposition or runaway conditions is key.
Previously noted by me in this other thread
http://www.sciencemadness.org/talk/viewthread.php?tid=10184&...
and here _
http://www.sciencemadness.org/talk/viewthread.php?tid=361&am...
HYDROGEN PEROXIDE HANDBOOK
quote :
Hydrogen Peroxide and its aqueous solutions also posses, in general,
solvent or solute relationships that are similar to water. The result
of several experiments show that sodium fluoride, potassium nitrate,
various potassium or sodium phosphates, potassium chloride, and
sodium or potassium sulfate are more soluble in H2O2 than in water.
Sodium nitrate, sodium chloride, silver nitrate, lead nitrate, and
lithium nitrate and sulfate are less soluble in H2O2 than water.
chlorine and iodine are only slightly soluble in anhydrous H2O2.
My note : valid for those salts which are near pH neutral or acid
since H2O2 is decomposed by basic substances. Low pH promotes
reaction with other materials in solution, dissolving Cu, Ag, Hg.
An interesting exception is the ClO3- ion which neutral or alkaline is
not reactive. ClO4- ion is not reactive at any pH and it is used in
Mg(ClO4)2 as a drying agent when concentrating H2O2.
It would appear that a peroxide solution of modest concentration perhaps
the highest available OTC spiked with some non-reacting oxidizer and
fueled with aluminum and suitable trace of an organic to sensitize,
can be detonable.
.
|
|
|
wolfy_9005
Harmless

Posts: 1
Registered: 29-3-2009
Member Is Offline
Mood: No Mood
|
|
If your going to use gelatine, pour boiling water over it, then add it to the h202. Maybe heat the h202 to around 50-60C then stir it in. The gelatine
usually comes in a sheet, and you need to soak these first. They should feel squishy, but wont fall apart if done correctly. Then drain as much excess
water off as possible, and add to the h202.
Or, make yourself some jelly - all you need to add is a flavouring 
And i think guar gum is used to make chewing gum chewy, so it may be mouldable, assuming it will stay in solution with the h202/etc and act like a
chewing gum, and not as a gel, per se.
Learnt this when i went to school to learn how to cook :p
|
|
|
hellfire23
Harmless

Posts: 16
Registered: 10-2-2009
Member Is Offline
Mood: bleh
|
|
Quote: Originally posted by wolfy_9005  | If your going to use gelatine, pour boiling water over it, then add it to the h202. Maybe heat the h202 to around 50-60C then stir it in. The gelatine
usually comes in a sheet, and you need to soak these first. They should feel squishy, but wont fall apart if done correctly. Then drain as much excess
water off as possible, and add to the h202.
Or, make yourself some jelly - all you need to add is a flavouring 
And i think guar gum is used to make chewing gum chewy, so it may be mouldable, assuming it will stay in solution with the h202/etc and act like a
chewing gum, and not as a gel, per se.
Learnt this when i went to school to learn how to cook :p |
With that method you will need a higher H2O2 percentage, to account for the water.
Also I found guar gum, about 3 grams, inside one of those kid friendly science kits. It was a slime kit.
|
|
|
| Pages:
1
2
3
4 |