| Pages:
1
2
3 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by Axt
On the structure of the "pseudonitrosites", its another one thats been restructured and renamed. As I said two posts up they have been given the
structure -NO=NO-, I just downloaded an article that gives them the name "azodioxy" compounds thus in recent literature that is what you would search
for. Ethylene being 1,1'-dinitro-2,2'-azodioxyethane. The articles attached.
It mentions the green colour of the C-nitroso compounds (my solution turned dark green) and the thermal decomposition of azodioxy compounds to the
C-nitroso compounds. The crystals I produced turned green on ignition thus good evidence that they were the targeted compound.
EDIT: "In general, the dimers are colorless, but the monomers are blue (aliphatic) or green (aromatic)" <i>Chem. Rev. 2002, 102,
1019-1065</i> Ok, mine should have been blue  oh well, maybe its more
complex then blue aliphatic, green aromatic. Though oxidation of the aromatic <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=5813">diaminofurazan</a> went through the green nitrosofurazan intermediate to
azoxyfurazan, hmmm where did that oxygen go oh well, maybe its more
complex then blue aliphatic, green aromatic. Though oxidation of the aromatic <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=5813">diaminofurazan</a> went through the green nitrosofurazan intermediate to
azoxyfurazan, hmmm where did that oxygen go 
[Edited on 12-5-2007 by Axt] |
DMSO is another possible alternative solvent for these reactions .
See US3822314 , example 16 .
This made me think of that experiment you did in the other thread also where you tried acetone peroxide
in xylene , but the xylene reacted also .
[Edited on 11-5-2007 by Rosco Bodine]
Attachment: US3822314 Preparation of Nitroximes and Nitroketones.pdf (133kB)
This file has been downloaded 1147 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
HNO3 on Acetone
According to US patent 5043488 slow addition of nitric acid to acetone produces
an unknown explosive compound of undetermined structure. A sample of the novel
product was placed on a hot plate and I quote :
" When the temperature reached 40 °C, the material decomposed violently with
copious evolution of smoke and flame indicating it to be an excellent explosive. "
Ohh yeah - just the thing to replace TATB huh 
Any thoughts on what this may be ? The only cited reference that is related is
Urbanski Vol I page 82 - 83
The two reagents are known incompatibles and products derived from normal
concentrations are very varied and complex, conditions of reaction affect the
processes enough to defy identification of specific pathways.
An example from this Material Safety Data Sheet warning
http://cameochemicals.noaa.gov/chemical/7198
" equal portions of acetone, nitric acid, and 75% acetic acid exploded 4 hours
after it was prepared and placed in a closed bottle."
This has a long history see the attached zip
From Hand-Book of Chemistry - 1855 page 7
Leopold Gmelin, Henry Watts
The Encyclopedia of Chemistry: Practical and Theoretical - 1862 page 31
James Curtis Booth, Campbell Morfit
This gives journal references
The Encyclopædia Britannica - 1911 page 762
Hugh Chisholm
_____________
Other references found in 1911 encyclopedia
A. Hantzsch and 0. Graul (Ber. 1898, 31, p. 2854)
described several series of salts of the nitrolic acids, with particular reference to
ethylnitrolic acid. They discriminate between the red or erythro-salts, which are
well crystallized, very explosive and unstable compounds, and which regenerate
the colourless nitrolic acid on the addition of dilute mineral acids, and the
leuco-salts, which are colourless salts obtained by warming the erythro-salts or
by exposing them to direct sunlight. These salts cannot be converted either into
the red salts or into the free acid. An intensely yellow acid salt is described, as
is also a very unstable colourless salt which could not be examined further owing
to its very labile nature. The following structural formulas are assigned to these
compounds: R CAN OH R.CCN(OK) > O R C N02K NO 2 N(:O) NO nitrolic acid;
erythro-salt; leuco-salt.
The acid salts are obtained by the addition of one molecule of alkali to two
molecules of the acid in concentrated alcoholic solution at a low temperature.
They are unstable compounds which readily split into the red salt and the free
acid on standing.
The pseudo-nitrols, RR':C(NO)(NO 2), may be obtained by the action of nitrous
acid on the secondary nitroparaffins; by the action of silver nitrite on such
bromnitroso paraffins as contain the bromine and the nitroso group united to the
same carbon atom (0. Piloty, Ber., 1902, 35, p. 3 0 93); and by the action of
nitrogen peroxide on ethereal solutions of ketoximes (R. Scholl, Ber., 1888, 21,
p. 508; G. Born, Ber. 1896, 2 9, p. 93). They exhibit an intense blue color when
in the liquid condition or dissolved in alkali and possess a very sharp smell. On
oxidation with chromic acid they yield dinitrohydrocarbons, and on reduction
with hydroxylamine (in alkaline solution) or with potassium sulphydrate give
ketoximes, RR': C: NOH (R. Scholl and K. Landsteiner, Ber., 1896, 29, p. 87).
* Note some may need to remove the .zip extension after downloading and click
to open, then select unzip program from the window menu to open. Next drag
the icon from inside the displayed folder out to the desktop and click that to
open and once again select the program from the window menu.
The contained .jpeg and .pdf should be visible then and viewable.
[Edited on 4-2-2008 by franklyn]
Attachment: pat5043488 Nitrated acetone.zip (464kB)
This file has been downloaded 1047 times
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The general character of the pseudonitrosites has been described by Wieland in Ann. 329, 225-268 (1903): colorless, not soluble in nearly any solvent
without decomposition (except CHCl3, which solubilizes most in the cold without decomposition). When warm they are solubilized by glacial acetic acid,
benzene, toluene, etc. forming nitrogen oxides, from acetic ester a small portion can crystallize unchanged. Upon storage they discolor to yellow
after a short amount of time, here nitrogen oxides and HCN are released. At 30-40 C, they decompose after a few days.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Quick post.
This looks exciting! I may test...
Ethene... I.E. EThylene.
Use Al2O3 as catalyst. We did it in chem a while back. I will draw a pic later.
Bollocks, someone got there first.
I will have to say though, it works a charm but beware of suckback. Suckback, or delivery tube melting and overpressure = boom caused some injuries in
that class. I left it with an idiot for a minute to get a gas jar when BOOM. The tube shattered in the face of the idiot. Nothing serious... Just a
scare
[Edited on 19-6-2010 by -=HeX=-]
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
http://pubs.acs.org/doi/abs/10.1021/ja00388a035
Reaction of nitrogen dioxide with alkenes and polyunsaturated fatty acids: addition and hydrogen-abstraction mechanisms.
So apparently there are two competing reactions,
2CH2CH2 + 3NO2 --> NO2CHCH2 + H2O + NO
CH2CH2 + 2NO2 --> (NO2)CH2CH2(NO2)
Is the second reaction reversible? How can I get the first to dominate?
What about CH3NO to make ONCH2NO ?
CH3NO can be made with NH2OH and CH2O.
"CH3NO → CH2NOH tautomerization"
pubs.acs.org/doi/abs/10.1021/jo051897r
Found some interesting alternate ways to put a nitro on an alkene, such as ethylene. Nitric oxide and "acidic Al2O3"
or use NaNO2, Ce(NH4)2(NO3)6, and glacial acetic acid.
The Nitro Group in Organic Synthesis ,By Noboru Ono
http://books.google.com/books?id=XmBZAvILOKAC&pg=PA13&am...
[Edited on 7-7-2010 by Anders Hoveland]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
QUOTE:
"Found some interesting alternate ways to put a nitro on an alkene, such as ethylene. Nitric oxide and "acidic Al2O3"
or use NaNO2, Ce(NH4)2(NO3)6, and glacial acetic acid."
It's been around in various patents, etc. One of the reasons (that I could think of) it's not common is that acetic acid/ (x)NO2 is generally a more
expensive route. However, if for some reason you have a healthy supply of acetic acid, you could try that with a variety of materials.
I had some acetic acid some years back & I know for a fact that it will function beyond the alkenes (nitroparaffins). Although temp control is an
issue as the acid will solidify (freeze) at a rather high temp.
EDIT:
As I remember the nitration was successful.
[Edited on 7-7-2010 by quicksilver]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  | I had some acetic acid some years back & I know for a fact that it will function beyond the alkenes (nitroparaffins).
As I remember the nitration was successful.
|
What exactly are you saying?
Do you mean you used glacial acetic acid to do a nitration with NO2 or KNO3 on an
alkane (ex. CH3CH3)? I would find this hard to believe.
If you mean a nitration inolving acetic acid and KNO3 on glycerine, then I would think this might be possible.
I do not think glacial acetic acid and 70% nitric acid would be compatible in a nitration procedure though.
Does anyone know exactly what "acidic alumina" means?
I found this: "There are three types of alumina that are available. The first type is acidic in character (pH 4.0)"
"Acidic Alumina
In chemistry laboratories, alumina is a medium for chromatography, available in basic (pH 9.5), acidic (pH 4.5 when in water) and neutral
formulations."
[Edited on 7-7-2010 by Anders Hoveland]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
I will have to post the patent (as well as notes). Rather there are a series of patents wherein materials other that the "classic mixed acids" were
used to nitrate various materials. I am not saying that it would not function w/ glycerin but those that I have seen, were not.
Indeed, there is an occasionally posted nitration of phenol to dinotrophenol using sodium nitrite, sodium hydroxide, 60/25% HNO3 (& of course
phenol). This indeed does produce product. It (the patent) was mistakenly reported as (application#) GB 365208 by Legard. Further investigation found
this to be a typo but the synthesis DOES produce a di-nitration. UK patent application dated may 30, 1973, David Anthony Salter, Robert Simkins.
70% HNO3 has been used OFTEN with glacial acetic acid in a common RDX synthesis:
glacial acetic acid
DAPT
ammonium nitrate
acetic anhydride
-=AND=-
70% HNO3
[William J. Sukasavage, Las Vegas NV Aug 28, 1992 application] Patent owned by US Army
EDIT
QUOTE:
"Does anyone know exactly what "acidic alumina" means?"
In what context was this used? It sounds like 19th century reference.
[Edited on 7-7-2010 by quicksilver]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2...
Methyl-Ethyl ketone MEK is readily available as a paint thinner. CH3CH2C(=O)CH3
Add NH2OH which will condense with the product in the picture to form the double oxime. CH3C(=NOH)C(=NOH)CH3
This should react with NO2 gas, and then be oxidized to
2,2,3,3-tetranitro butane
This would be powerful, but might be moderately sensitive.
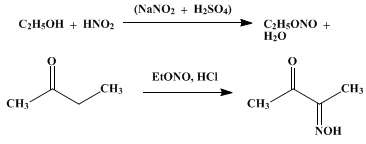
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Yes this reaction is known. Sadly the yields from the oxidation step seem to be low in literature.
I was going to say it would be interesting to try with phloroglucinol trioxime to make 1,1,3,3,5,5-hexanitrocyclohexane but I found literature saying it instead forms hexanitrobenzene.. Maybe this is a useful synthesis of tetranitrobenzene? The yields would probably be bad
though.
You might be able to nitrosate cyclohexane-1,4-dione and react with hydroxylamine to get 1,2,4,5-cyclohexanetetroxime. Then to
1,1,2,2,4,4,5,5-octanitrocyclohexane? Too unstable I suppose.
[Edited on 8-7-2010 by 497]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  | there is an occasionally posted nitration of phenol to dinotrophenol using sodium nitrite, sodium hydroxide, 60/25% HNO3 (& of course phenol).
70% HNO3 has been used OFTEN with glacial acetic acid in a common RDX synthesis: glacial acetic acid, ammonium nitrate,
acetic anhydride, 70% HNO3
|
Did you mean that sodium nitrite and anhydrous dry NaOH alone is capabable of nitrating phenol? If this is the case, I have seen amine groups being
added to trinitro benzene by NH2OH and fused KOH used to dehydrate/ make the amine condense on (forming trinitro, triamino benzene) in moderate yield.
Or did you mean that both nitric acid and NaOH were used in the nitration? Why use the NaOH?
70% HNO3 is used in the RDX with acetic acid, but the nitric acid is slowly added, and it immediately dilutes itself in the acetic acid or reacts to
form the nitramine before the concentration gets high enough to start oxidizing the acetic acid. I know from experience that 5% HNO3 solution is
incapable of dissolving copper foil after 2 days, but I suspect that the copper would eventually dissolve if given enough time. The more HNO3 is
diluted, the less the equilibrium favors the oxidizing nitronium ions.
Quote: Originally posted by 497  |
it would be interesting to try with phloroglucinol trioxime to make 1,1,3,3,5,5-hexanitrocyclohexane, but it instead forms hexanitrobenzene.. Maybe
this is a useful synthesis of tetranitrobenzene? The yields would probably be bad though. |
I do not think there is any particular reason why the yields would not be very high, whatever byproducts would form would probably be stuff like
1,1,3,3,5,6-hexanitro cyclohexene, the molecule containing only one double bond between two carbon atoms. The mix of products need not necessarily be
separated from eachother to be useful. The 1,4-oxime of quinone could likely react with NO2 to make 1,4,-dinitro, 2,5-dinitroso benzene, which when
oxidized would afford the tetranitro. Or instead, be reduced with bisulfite to 1,4-dintro, 2,5-hydroxylamino benzene that could be used for energetic
salts. Or tetranitro benzene heated with NaN3 would make the 1,2,4,5-di furoxan of benzene
(NaN3 used in 'Megalomania's synthesis of 'Cl-22'), which could then have another nitro group easily added. All these routes are probably obvious to
most readers.
Quote: Originally posted by 497  |
You might be able to nitrosate cyclohexane-1,4-dione and react with hydroxylamine to get 1,2,4,5-cyclohexanetetroxime. Then to
1,1,2,2,4,4,5,5-octanitrocyclohexane? Too unstable I suppose.
|
Was a typo mistake was made? If you nitrosated cyclohexane-1,4-oxime (with NO+ cations) you would likely get 1,2,4,5-tetra nitroso benzene. If you
reacted cyclohexane-1,4-dione with NO and NO2, then up to two NO or NO2 groups would likely be added to positions
#2,3,5,and 6. This would be random. Condensing this with NH2OH, and then using NO2 on the resulting oxime, and finally oxidizing any nitroso groups to
nitro, would presumably give a final product of poly-nitro hexane. I am fairly certain that anything pass 6 nitro groups would be highly unstable.
What do you mean "nitrosate cyclohexane-1,4-dione and react with hydroxylamine"? I do not understand.
1,1,2,2,4,4,5,5-octanitrocyclohexane would probably be just a little more stable than nitroglycerine, and more powerful than HMX.
[Edited on 8-7-2010 by Anders Hoveland]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
A long time back; certainly 4 years ago, there was a long thread that detailed all the things one could do with 70% HNO3; all the nitration, the labs
(fulminate), etc, etc.....When you first started writing about 70% acid I looked for that but couldn't find it. Additionally I don't know if deletions
take place to maintain a certain data-size footprint on the forum, but I do remember the thread.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | What do you mean "nitrosate cyclohexane-1,4-dione and react with hydroxylamine"? I do not understand. |
Sorry, I didn't explain very well there. I meant do the style of nitrosation that adds an oxime adjacent to a ketone, as mentioned earlier in the
thread. I know that doing that on cyclohexanone will yield 2-oximinocyclohexanone, and I am assuming the it would also occur on both sides of
cyclohexane-1,4-dione to yield 2,5-dioximinocyclohexane-1,4-dione.. maybe also the 2,3-dioximinocyclohexane-1,4-dione but I'm guessing that is less
likely. The treating the remaining ketone with NH2OH to yield the 1,2,4,5-tetraoxime... from there treat with NO2 then H2O2 as usual to hopefully end
up with the octanitro product. My logic is, if it works for dimethylglyoxime, it should work here.. Also, I know 1,1,4,4-tetranitrocyclohexane
exists..
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
It all seems like it might work, but the big disadvantage is: how would you make the
dioximinocyclohexane-1,4-dione? This does not seem to be within the capabilities of even the most enthusiastic of basement experimenters.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Anders: I know a few companies that will custom - synth chemicals for you, as long as the chemical is not illegal. No questions asked at all. They
deliver to home and all  Sadly, it seems to be Europe only Sadly, it seems to be Europe only
That puts it in reach... Though perhaps not of my budget. Just wait till I get *that* job 
As for the OT hexanitrobenzene--->tetranitrobenzene, how do we strip off the Nitro groups and put Hydrogen on? I know a reduction (partial) would
only cause it to be, at closest, Tetranitro-diaminobenzene
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | It all seems like it might work, but the big disadvantage is: how would you make the dioximinocyclohexane-1,4-dione? |
I'm a little confused as to what you mean.. The dioximinocyclohexane-1,4-dione would be produced by treating cyclohexane-1,4-dione with a mixture of
isopropyl nitrite, HCl and DMSO for example, although there are other reagents that could be used.
If you meant, where would the cyclohexane-1,4-dione precourser come from, well that is a bigger problem. It might be easiest to go hydroquinone ->
p-benzoquinone -> p-benzoquinone diketal, then hydrogenate that to cyclohexanedione diketal, which could be directly nitrosated since the ketals
would hydrolyze in situ. There are references for basically all the steps, and yields should be good in all steps except the final oxidation steps.
The hydrogenation would be the only problematic step since it would require a catalyst and probably pressurized hydrogen.. But home made Urushibara
nickel catalysts are really easy. Add in a little hydrogen from a home made electrolysis cell and it could be done. No exotic reagents required.
Man if it was stable enough I bet the octanitrocyclohexane would be a really kick ass explosive. Near perfect OB. And of course there are always
possibilities for adding even more power/stability by substituting the two unsubstituted carbons with something.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I do not think it would be an ideal explosive. The chemist would have little control over exactly where the nitro groups would be added to the
molecule, so some of the molecules might have all the nitro's clustered toward one end, some molecules might have only one or two nitro groups, and
others might have way too many. This would give the mixture unnecessary instability and extreme sensitivity if it was be nitrated enough to be
significantly powerful. I think it is probably too complex to be very useful. Consider instead other stuff like trinitro ethane.
If you have access to CH3CH2CH=O then you could condense it with NH2OH, then bubble in NO2, then possibly oxidize with H2O2 (if that would work) and
get 1,1,1-trinitro propane.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Axt  | From the attachment three posts up.
"Franklin and Willtins (61) believed they obtained 1,4-dinitro-2-butene from the reaction of nitrogen tetroxide and 1 , 3-butadiene when these
substances were allowed to react in various solvents or in the vapor phase."
So, CH2=CH-CH=CH2 --> O2N-CH2-CH=CH-CH2-NO2
Same goes with all other dienes it that article, they retain a double bond. |
Does anyone alive today have a copy of or know the reference for "the attachment three post above" as I am trying to find the Franklin and Willtins
reference here but I can't find any Willtins in the author citation and wondered if it is a spelling mistake
|
|
|
| Pages:
1
2
3 |
|