| Pages:
1
2
3
4
5
6 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Checking my calcs on the dissolution of Tb in HCl 37 w%, it works out (as above) for one mole of Tb you need 296 g of HCl 37 w% (stoichio amount, in
reality you need a bit more, of course), giving 1 mole of TbCl3 dissolved in 186.4 g water, or 10.3 mole of water. The mole fraction
X<sub>T,S</sub> of TbCl3 in solution is thus 1 / (1 + 10.3) = 0.088, post reaction.
From here can also be estimated how much of the TbCl3.6H2O will crystallise out on cooling to RT, where X<sub>T,S</sub> = 0.06, from:
n<sub>T,C</sub> = [(n<sub>T</sub> + n<sub>W</sub> . X<sub>T,S</sub> - 1] / [(1 + 6) . X<sub>T,S</sub> - 1 ] . X<sub>T,S</sub> - 1] / [(1 + 6) . X<sub>T,S</sub> - 1 ]
With:
n<sub>T,C</sub> number of moles of TbCl3 in the solid (crystals) phase
n<sub>T</sub> total number of moles of TbCl3 in the system (i.e. 1)
n<sub>W</sub> total number of moles of water in the system (i.e. 10.3)
X<sub>T,S</sub> = 0.06, mole fraction of TbCl3 in solution
This works out at n<sub>T,C</sub> = 0.555
So even without chilling, at about RT, more or less half of the TbCl3 should crystallise out as TbCl3.6H2O. Not bad at all...
[Edited on 6-12-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
An interesting finding
Warning: long post.
Quote: Originally posted by blogfast25  |
Can I insist on a short version of a write up? Tip: always save something longish in your favourite word processor before submitting here.
I'm surprised it seems to hydrolyse so easily and am wondering if you're perhaps seeing something else. If there's a next time, try acidifying the
solution a little with acetic acid (I'm assuming the acetate is very soluble, so won't crystallise when you want to try and recrystallize the
TbI<sub>3</sub> . .
...
To dissolve the Tb in the acid, add small lumps of the metal to the acid, expect much effervescence and heat. Cool between additions if needed
(probably, at least at first!)
And take pictures! You don't get to see that kind of thing everyday!
[Edited on 5-12-2013 by blogfast25] |
Tip taken. And the write-up will be included.
Terbium halides (and most other lanthanide salts) hydrolyze very easily on heating. The thing is, I boiled the solution very quickly, and a lot of
terbium iodide remained in solution.
Well, you read my mind about the TbCl3. I've already done the reaction and taken pictures. You won't see this in the picture, but at high
concentrations of Tb3+, the solution becomes yellow; it's the color seen in Pok's post in versuchschemie. I only used 1M HCl and got the result. 37% HCl should show an even stronger yellow. I don't speak German, but from
what I understood from a machine translation, the color was thought to be a contamination. It seems to be inherent to the terbium(III) ion at high
concentration. I need to test this solution - it may have some of the absorption band effects of other rare-earth salts.
So here's what I did:
A small test was done with a few small bits of terbium added to a test tube. A large excess of iodine was added in the expectation that most of it
would vaporize. The test tube was heated, and a huge puff of iodine vapor formed, with what appeared to be iodine droplets rolling down the test tube.
Note to blogfast25: I don't have any fancy vacuum stuff or argon, so I just heated them together. There was oxygen in the tube, but terbium seems
unusually reluctant to react with oxygen outside of a flame (I just heated terbium in a test tube and there was no visible reaction, but it burns
readily, similarly to burning cerium).
At the bottom of the test tube, some stuff had accumulated. It looked like a salt, but had a metallic sheen. I don't know if this was terbium iodide
or just iodine - the color was not clear. It may have been terbium triiodide (which refers to Tb(I3)3, just to clear up any
confusion). Unreacted iodine coated the test tube.
In order to test if the terbium had actually reacted to produce terbium iodide, I dissolved the solution in water. I forgot to test this solution for
fluorescence. No insoluble particles were suspended (so likely no oxides formed), but the solution became very dark, likely due to triiodide
formation. The solution was heated in the test tube, and it bubbled violently (a test with iodine aqueous solution only showed light bubbling and
iodine vapor emission).
After removal, the solution had become lighter and mixed terbium oxides precipitated. This solution is the one that fluoresced.
In the fluorescence trials, I've discovered something very interesting.
A test tube with only iodine solution in water shows no fluorescence and no green color in the light. A test tube with terbium chloride also shows no
fluorescence - it looks absolutely clear.
The test tube that fluoresced contained a mixture of terbium iodides and triiodides, with precipitated terbium(III) and terbium(IV) oxides. In order
to rule out suspended oxides and hydroxides as the source of the fluorescence, I added 1M HCl to a different test tube containing the solution. The
oxides dissolved and the fluorescence was still observed, and looked much clearer due to the lack of mixed oxides.
So this implies that the fluorescence is due to an iodine species - either iodide, triiodide, or possibly iodate. I'll report back soon. My next test
will be to add KI or NaI to TbCl3 and check for any fluorescence.
Some non-experimentation notes:
MrHomeScientist - Thanks for replying! Your posts and video inspired my project.
I don't have a YouTube account, so I'll tell you this here - the black stuff that formed in the terbium nitrate synthesis is a mixed terbium(III,IV)
oxide, similar to manganese dioxide. The stuff appears to be a good catalyst for the ignition of coal gas and the contact process for sulfuric acid
production. I hope you kept it - it's not a waste product by any means.
blogfast25 - I made a mistake when I said I cannot access the paper; I meant that the link is broken.
And thanks for the calculations, they are pretty comprehensive.
tl;dr: Stuff worked.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
B&F:
Firstly, if I ever mentioned vacuum in the context of Tb/I<sub>2</sub> then I must have been foolish: only Argon would work here because
the iodine is too volatile.
Secondly, acc. wiki, solid terbium sulphate strongly UV fluoresces green. All RE sulphates are basically very poorly soluble in water, so adding
sulphuric acid to any RE solution will precipitate them. Worth doing if the fluorescence is what you want to study.
Also, the double salts RE<sub>2</sub>(SO<sub>4</sub> <sub>3</sub>.K<sub>2</sub>SO<sub>4</sub>.nH</sub>2</sub>O (n is 2 or 3, can't remember right now)
are very poorly soluble and can be used to separate REs from 'stuff' or just to recover odds and sods of RE solutions. This is done by saturating the
RE solution with potassium sulphate. <sub>3</sub>.K<sub>2</sub>SO<sub>4</sub>.nH</sub>2</sub>O (n is 2 or 3, can't remember right now)
are very poorly soluble and can be used to separate REs from 'stuff' or just to recover odds and sods of RE solutions. This is done by saturating the
RE solution with potassium sulphate.
Both the sulphate and the double sulphates can be turned back into hydroxide by treatment with strong NH3. The sulphate, ammonia/ammonium and
potassium can then be washed out easily and the RE(OH)3 redissolved in the acid of your choice. I've done this quite a few times by now with Nd.
Dissolving fairly dry RE(OH)3 (suction dried, for instance) in strong HCl is another way of preparing a strong solution of RECl3.
Lastly, it's possible your terbium is contaminated: it's not supposed to display the mechanical properties you described. A test for iron (III) with
thiocyanate could be useful, as Fe is a common fellow traveller of REs. Incidentally, the yellowish colour of Fe(III) might even explain the yellow
you mentioned (although it would take rather a lot of Fe and it would first form as Fe(II)). So it would be strongly recommended to rule out Fe with K
or NH4 thiocyanate solution, for peace of mind.
[Edited on 7-12-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
The vacuum thing was my creation, sorry about that.
What I found interesting is that the fluorescence of chlorides (and according to MrHomeScientist, nitrates) doesn't show up in solution. The problem
I'm starting to have with my theory of iodide complexing with terbium to form some other ion in solution is that iodide is not a very good complexing
agent, and would likely have a higher free energy of formation than, say, chloride, or even water itself.
I'll do the thiocyanate test for iron when I can experiment. I don't know if I can get ahold of thiocyanate, though. Metallium gives a minimum purity
of 99.5% for terbium, so I doubt that there's a significant amount of Fe in the sample.
I've noticed that my project has strayed significantly from its original intent - studying triboluminescence of hexakis(antipyrine)terbium(III) iodide
and substituted derivatives. Part of the difficulty (and the interesting results) have arisen solely from the synthesis of terbium iodide (and I still
have to find a way to get rid of excess iodine without hydrolyzing the product).
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Brain&Force  | [...], and would likely have a higher free energy of formation than, say, chloride, or even water itself.
[...]
Metallium gives a minimum purity of 99.5% for terbium, so I doubt that there's a significant amount of Fe in the sample.
|
The free energy of formation values of most simple ions in water are tabled, so just search. But I'm not sure how this is to impact complexing? TbI3
in water shouldn't be complexed I think...
0.5 % impurity could be enough to make the metal harder than the pure substance. Iron is quite a common contaminant, that's why I suggest testing for
it.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
More findings
More fun results...
The solution that "fluoresced" actually didn't. It was caused by triiodide ions in solution. As the concentration of triiodide in solution increases,
the UV part of the spectrum fades, and the remaining visible spectrum becomes apparent. I'm using an LED diode.
blogfast25, I tried the thiocyanate test and I got a negative result, with the solution remaining almost clear (barely yellow from Tb). A very dilute
solution of ferric sulfate (I don't know exactly how much, it was just a few grains) gave a blood red color. Now I know what I'm doing for Halloween
next year...
The solution has a very slightly greenish yellow tint with no turbidity. Addition of a few drops of HCl solution causes the complex to become
perfectly transparent. It's very odd and has nothing to do with dilution. My best guess is that a colorless complex [TbCln]3-n
exists in solution, and with high concentrations of Tb3+, the cations form yellow some-number-aquaterbium(III) ions. (Terbium can vary its
coordination number from 6 to 9) The concentration of terbium in solution can't be greater than 0.33 molar (I started with 1M HCl). How could I
determine the composition of a complex ion in solution?
Of course, I could be wrong, feel free to suggest your theories.
This complex MAY be fluorescent. I can't be sure; I'm attempting to make more TbCl3 and getting a better UV light.
Note: I made a larger amount of the yellow stuff, but I unintentionally destroyed it with HCl.


At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
B&F:
I've never really heard of coordination complexes of the f-block elements. Most d-block elements that form them are amphoteric to some extent and the
REs aren't amphoteric. But I could be wrong on complexes. Seems to me the f-orbitals don't lend themselves to bonding ligands very well. Again, I
could be completely off the mark here. Will google a little later on.
What's happening in pic 1? Tb in HCl?
[Edited on 13-12-2013 by blogfast25]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  | B&F:
I've never really heard of coordination complexes of the f-block elements. Most d-block elements that form them are amphoteric to some extent and the
REs aren't amphoteric. But I could be wrong on complexes. |
Most transition metals will form complexes- you just have to pick the right ligand. Right off the top of my head, copper, nickel, silver and iron
aren't amphoteric (I've heard that copper is, but the solution must be screamingly basic for it to show any sign of being so), but readily form
complexes with ammonia, ammonia, ammonia and halide ions (respectively).
For the lanthanides, Cotton & Wilkinson tell me that the most stable complexes are those with oxygen-binding ligands (EDTA, citrate, tartrate,
carbonate, acac, etc.), and the most common coordination numbers are 7, 8, and 9.
Relevant to this discussion is the following tidbit (C&W, Advanced Inorganic Chemistry, 4th ed., p 992):
"Halogeno complexes. In aqueous solutions rather weak complexes MF2+(aq) are formed with fluoride ion, but there is little evidence for
complex anion formation; this is a distinction as a group from the actinide elements, which do form complexes in strong HCl solutions. However by the
use of nonaqueous media such as ethanol or acetonitrile, salts of the weak complexes MX63- can be prepared. The iodo complexes
are exceedingly weak, dissociating in nonaqueous solvents even in the presence of an excess of I-, and they are attacked by moisture and oxygen."
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
DA:
Thanks. Cu is definitely amphoteric (can be shown quite easily at home level) and Fe forms (instable) ferrate (VI). But I agree it's not a very strong
correlation.
In your quotation, does M stand for a lanthanide? I had certainly forgotten about EDTA as a complexing agent for REs. EDTA complexometry is one way of
determining RE solutions.
But RE chloride complexes: probably not. And certainly not iodo complexes.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  | In your quotation, does M stand for a lanthanide? I had certainly forgotten about EDTA as a complexing agent for REs. EDTA complexometry is one way of
determining RE solutions.
But RE chloride complexes: probably not. And certainly not iodo complexes. |
Yes- that's from the section on lanthanides. Chloride complexes- only in nonaqueous solvents, iodo complexes, even then, just barely. They will also
form complexes with chelating N-donors (en, etc.), and thiocyanate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I was able to dry some TbCl3 in a vacuum dessicator, but only partially. It displayed a strong yellow color, similar to that of a
fluorescent highlighter. It looks the same in sunlight and tube lighting. (I'll need to try CFL lighting as well because that produces the most
dramatic color shift.) However, no fluorescence could be observed. I have a feeling water may be able to quench the fluorescence of terbium compounds
- after all, terbium chloride is supposed to fluoresce. I'll test nitrate as well.
blogfast25: Pic 1 is Tb in HCl. The complex isn't visible until after the HCl solution is depleted.
Is it possible that this yellow color is TbOCl (terbium oxychloride) forming? This may be possible, given the complex appears to turn clear in even
the slight amount of acid. If this were a chloro complex of terbium, I would expect the color to change only slightly as progressively larger amounts
of HCl are added. I haven't tried redissolving in pure water - maybe that will confirm the result.
From woelen's site, praseodymium oxychloride appears to be only slightly, if at all, soluble in water. This complex is soluble in water - so either
TbOCl is soluble or there's something else going on.
Perhaps the answer may lie in this thread? Perhaps different preparations can produce different colors.
Pic 1: complex after drying. It was still wet, I'm going to vacuum it some more.
Pic 2: I decided to dissolve it in a 0.3 molar TbCl3 solution with some excess HCl, originally clear, and the solution turns very pale
yellow.
 
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I dried the solution further and ended up getting yellow flakes of what appears to be terbium(III) chloride. Again, it should be white, but I have no
clue as to what's causing the yellow color. There was iodine in the dessicator after a spill occured, but that can be ruled out because the color of
the solution was yellow to begin with. A test for fluorescence came up mostly negative, but there were a few flakes that glowed greenish. The rest
took on a very dim yellow color. (I've heard dysprosium has yellow fluorescence, but I have no idea if there really is any.) As expected, the flakes
are paramagnetic, but only slightly.
In CFL light, the color does not change at all. It's still yellow.
Oddly enough, it smells like oranges. This is really unusual - the compound shouldn't be volatile at all. The only volatile compound I could fathom
exists would be terbium(IV) chloride, but I doubt HCl can produce this. I can't be sure if it's the compound itself or some volatile substance that
was in the vial earlier.
Now I need to figure out what this yellow stuff is...
Here are two photos of the compound - one it normal light, the other in UV. During the vacuuming, some glass in the dessicator chipped off, and there
are fragments on the watch glass.
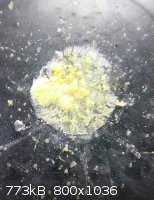 
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
B&F:
I doubt if TbOCl2 can form in these conditions. These halides are just not that prone to hydrolysis.
How pure is your HCl? Iron is very common in garden variety strong HCl. That picture looks so much like some white chlorides I've prepared
with hardware store 'muriatic acid'!
Try also to add H2SO4 to the solution to see if the sulphate (hydrate) precipitates and whether it fluoresces (strongly green under UV, acc. Wiki).
Can I borrow (with credit to you) that picture of Tb dissolving in HCl?
[Edited on 21-12-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
You can use the picture. Where is it going to be used? If you need a larger one, I can send you the full size version.
The hydrochloric acid is not hardware store acid - it's 1.0 molar acid, source unknown, but definitely from one of those large chemical suppliers. I
made Tb(NO3)3 and it still showed the same color. I also added hydrogen peroxide and tested for thiocyanate so that any
Fe2+ would be oxidized to Fe3+, but there is still no red color.
[edit] I've ruled out the following as contaminants:
Iron, lanthanum, praseodymium, neodymium, holmium, erbium, thulium, ytterbium, lutetium.
These are possibly contaminants:
Cerium, samarium, europium, gadolinium, dysprosium (they all form salts that have some sort of yellow tint).
I think cerium(IV) may be a contaminant in my sample - cerium(IV) sulfate is yellow, so other cerium salts may be mixed. However, I highly doubt
CeCl4 is stable.
Any suggestions?
[Edited on 23-12-2013 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ce(IV) oxidises Cl<sup>-</sup>, IIRW.
I'll use the photo on my blog.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
There's an interesting finding I forgot to mention in this thread. In the solution that appeared to fluoresce but didn't, there were terbium(III) and
triiodide ions, and terbium(III) oxide had precipitated out. Over the next few days, the brown color faded to yellow, and eventually the color became
clear. Apparently iodine is strong enough to oxidize terbium(III) oxide to terbium(III,IV) oxides.
I'll make some Tb2O3 and see if I can repeat it.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
That is of course only one possible explanation but not very plausible IMHO.
From my latest gadget, the CRC Ed. 86:
Tb<sup>3+</sup> === > Tb<sup>4+</sup> + e, E<sub>Ox</sub> = - 3.1 V
I<sub>2</sub> + 2 e === > 2 I<sup>-</sup>, E<sub>Red</sub> = + 0.5355 V
This makes oxidation of Tb(III) to Tb(IV) by iodine very unlikely.
Is it possible that some I<sub>2</sub> or I<sub>3</sub><sup>-</sup> got chemisorbed onto the surface of the
oxide/hydroxide and that the iodine later simply oxidised by air (to hypoiodate/iodate)?
[Edited on 26-12-2013 by blogfast25]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  |
From my latest gadget, the CRC Ed. 86:
Tb<sup>3+</sup> === > Tb<sup>4+</sup> + e, E<sub>Ox</sub> = - 3.1 V
I<sub>2</sub> + 2 e === > 2 I<sup>-</sup>, E<sub>Red</sub> = + 0.5355 V
This makes oxidation of Tb(III) to Tb(IV) by iodine very unlikely. |
You can't apply solution potentials to oxides.
A half reaction such as Tb(OH)3(s) + OH- -> TbO2(s) + 2 H2O + e- will have a potential completely unrelated to the
one you gave above.
[Edited on 26-12-2013 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
DA:
I wouldn’t say ‘completely unrelated’. Break it down:
Tb(OH)<sub>3</sub> < == > Tb<sup>3+</sup> + 3 OH<sup>-</sup>…. (1)
Tb<sup>3+</sup> === > Tb<sup>4+</sup> + e …. (2)
Tb<sup>4+</sup> + 4 OH<sup>-</sup> === > TbO<sub>2</sub> + 2 H<sub>2</sub>O …. (3)
To the value for (2) will have to be added terms to account for (1) and (3).
[Edited on 26-12-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
The fact that terbium forms mixed oxides (Tb4O7 and Tb6O11) may complicate matters further. However it's
not known whether the compound is stoichiometric (like aluminum oxide) or interstitial (like manganese dioxide, which this oxide strongly resembles).
Pure TbO2 can't be prepared easily - you have to either expose it to atomic oxygen or reflux it with a solution of HCl in acetic acid.
Also, WebElements gives potential of Tb(OH)3 + OH- → TbO2 + e- as -0.9. Reduction is possible, but
probably not with I2. Interestingly, WebElements gives potentials for DyO2 and HoO2 (I can't find any references to
these compounds).
However, the fact that no soluble terbium(IV) compounds form may drive equilibrium to the right in this case.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Actually, the formation of ionic solids would drive it to the right, because of the release of lattice energy (although it also decreases entropy).
And if no solids are formed (Tb(IV) in solution) then surely the value for (2) stands?
Whether iodine can or cannot oxidise Tb(III) to whatever will need to be determined experimentally, as you suggested. Not easy, since as iodine is
itself prone to oxidation (in alkaline medium). I would mix a sub-stoichio amount of KI<sub>3</sub> solution with
Tb(OH)<sub>3</sub> and see...
Intuitively though, the more I think about it the more the oxidation of Tb(III) with iodine sounds implausible.
[Edited on 27-12-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I found an article, written in 1905, that details several terbium compounds - namely, the chloride, bromate, perchlorate, carbonate, citrate, meconate,
formate, acetate, and carbide. They didn't have ion exchange technology, so I'm guessing there were some significant impurities (likely LREEs) in the
samples. Nevertheless, it describes terbium chloride as producing a clear and colorless solution. I'll try to find some cleaner HCl to continue
experimentation.
I got some permanganate and citric acid for Christmas, so I'll test out two things:
Whether permanganate can oxidize Tb2O3. If iodine can, then permanganate should be able to as well.
Whether terbium citrate is fluorescent - and determine its color.
Is it possible for iodine to have contaminated the TbCl3? It looks similar to some polyhalides. I have some in a vial, so I'll leave it uncapped and see what happens.
And apparently neodymium can form a complex with HCl (scroll to the bottom).
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
B&F:
If by Tb<sub>2</sub>O<sub>3</sub> you mean Tb(OH)<sub>3</sub> , then permanganate should not be able to oxidise
it. For one, the hydroxide can only exist in neutral/alkaline conditions and in these conditions the reduction of permanganate leads to the dreaded
MnO<sub>2</sub>(s).
In acid conditions Tb would exist as Tb<sup>3+</sup> but the reduction potential of Mn(VII) === > Mn(II) is then + 1.51 V, not enough
if my value of – 3.1 V is correct. But it’s worth trying because its colourimetry in action! But you have to make sure you've got the quantities
right because permanganate is so intensely coloured it can obscure everything. Excess, unreacted permanganate could lead to erroneous conclusions
If your HCl is Fe(III) free then it should be good enough for your purposes. You can ‘recondition’ any contaminated ods and sods of Tb solutions
by first filtering them, then precipitate as hydroxide from dilute solution with ammonia solution, filter off hydroxide, wash filter cake profusely,
then redissolve in minimum amount of HCl.
Re, Nd/HCl complexes, where does he write that? All I can find is the fairly vague:
”Although the lanthanide elements are said to have much less pronounced color shifts due to complex formation, when compared with the transition
metal elements, the effect of a small shift is much stronger.”
Re, polyhalides, that's a good find. I didn't know they were so easy to prepare. So it can't be excluded out of hand that some
ICl<sub>4</sub><sup>-</sup> was formed...
[Edited on 28-12-2013 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Hmm...I forgot MnO2 would form. Considering the similarity of Tb4O7, it wouldn't be easy to tell if something else
had oxidized the permanganate.
But DraconicAcid has a point about oxide reduction potentials. The potential Fe2+ + 2e- → Fe is -0.44 V, but the potential
for Fe(OH)2 + 2e- → Fe + 2OH- is -0.89 V. These should seem related, but they're quite different.
Terbium(III,IV) oxide is a lot more stable than the reduction potential suggests. I believe Tb4O7 forms when terbium is burned,
but I'll have to test again.
The Nd-Cl- complex is mentioned on the bottom; there is a preparation of NdCl3 with dilute HCl and concentrated HCl.
I came up with this reaction to produce ICl4-:
I2 + 4Cl- → ICl4- + 3I-
so likely not much ICl4- has been formed. But this appears to be sufficient to color the crystals yellow.
The polyhalide theory explains a lot:
Why the compound smells. Strangely, the smell does not resemble iodine or chlorine - it's similar to tangerines. I'm thinking the large terbium cation
can stabilize this ion to the point that it does not decompose much. That would also explain why there is no change in color after leaving the bottle
open overnight.
The unexpected yellow color with relatively pure HCl.
I got the idea from this thread. I had been storing iodine in the fume hood with the test tube rack. Won't be making that mistake again.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Your point about the differing reduction potentials boils down to what I wrote above:
Quote: Originally posted by blogfast25  |
Tb(OH)<sub>3</sub> < == > Tb<sup>3+</sup> + 3 OH<sup>-</sup>…. (1)
Tb<sup>3+</sup> === > Tb<sup>4+</sup> + e …. (2)
Tb<sup>4+</sup> + 4 OH<sup>-</sup> === > TbO<sub>2</sub> + 2 H<sub>2</sub>O …. (3)
To the value for (2) will have to be added terms to account for (1) and (3).
|
The oxidation potential corresponds to a change in Gibbs Free Energy, ΔG. In the case of oxidation of Tb(III) to TbO<sub>2</sub> (or
a mixed oxide), Hess’ Law allows to split it into three terms:
ΔG = ΔG<sub>1</sub> + ΔG<sub>2</sub> + ΔG<sub>3</sub>
ΔG<sub>1</sub> will be positive, because it’s the precipitation of the hydroxide that is spontaneous, not dissolution.
ΔG<sub>2</sub> corresponds to the oxidation of ‘naked’ Tb(III).
ΔG<sub>3</sub> is likely to be strongly negative because it involves the spontaneous precipitation of an ionic solid.
On balance, ΔG is probably more negative than ΔG<sub>2</sub> alone, making the oxidation thermodynamically more
favourable, done that way.
It would be worth trying simple thin bleach: hypochlorite is a very powerful oxidising agent, in alkaline conditions. And no MnO2 in sight…
A better analogy would be Fe2+/Fe3+ and Fe(OH)2/Fe(OH)3. Or even Fe2+/Fe(OH)3. Ferrous stuff does oxidise much faster in neutral to alkaline
conditions, I think...
[Edited on 28-12-2013 by blogfast25]
|
|
|
| Pages:
1
2
3
4
5
6 |
|