| Pages:
1
2
3 |
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
xaxaxaxaxa!! realy funny!!  
[Edited on 14-2-2014 by underground]
|
|
|
Gargamel
Hazard to Others
  
Posts: 166
Registered: 9-3-2013
Member Is Offline
Mood: No Mood
|
|
Explosive performance of nitroguanidine
If you have guanidine nitrate, nitroguanidine could easily be prepared.
Apart from being a useful raw material for a lot of other things and also as a propellant:
How would it perform as a high explosive for shaped charges and other interesting experiments?
I found velocity figures from 6750 to 8200m/s, that sounds useful, even though it's not as energetic as the more common HEs.
I wonder how it behaves in practice concerning the typical problems one encounters, like achieving the right density, critical charge diameter and
sensitivity to initiation.
Any practical experience?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
One major problem with guanidine nitrate (EDIT: typo, meant nitroguanidine, thanks philou!) is that its morphology (needles) makes it very difficult
to get anything approaching a reasonable density for application in shaped charges.
Better to turn it into 5-AT, and make the nitrate salt. That has around 8800 m/s and density 1.8 if I remember correctly.
[Edited on 20-12-15 by The_Davster]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@ The_Davster,
Nitroguanidine, not guanidine nitrate!
From Explosives 4th revised and extended edition, Josef Köhler and Rudolf Meyer
p248 and 249
Nitroguanidine (H2N)2C=N-NO2
MW: 104.1 g/mol
Enthalpy of formation: -213.3 kcal/kg
OB: -30.7%
%N: 53.83%
Volume of detonation gases: 1075 L/kg
Heat of explosion (H2O gas): 687 kcal/kg
specific energy: 98 mt/kg
density: 1.71 g/cm³
melting point: 232°C = 450°F (decomposition without deflagration)
lead block test for 10g: 305 cm³
VOD at max density and confinement: 8200 m/s
impact sensitivity: up to 49 Nm --> no reaction
friction sensitivity: up to 353 N pistil load --> no reaction
critical diameter of steel sleeve test: 1 mm --> no reaction ... Thus very insensitive even if confined!
Soluble in hot water but almost unsoluble in cold water. Sparingly soluble in alcohol and insoluble in ether.
Readily soluble in alkali (formation of salts NQ is a nitramine NH2-C(=NH)-NH-NO2).
Excellent chemical stability and not very sensitive to shock or impact.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
thanks! I typed the wrong compound
|
|
|
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Does anyone know the properties of aminoguanidine nitrate ? Aminoguanidine can be made by reduction of nitroguanidine. Then just neutralize it with
HNO3
Nitroguanidine looks to be an interesting nitramine
[Edited on 20-12-2015 by underground]
|
|
|
Bert
|
Threads Merged
20-12-2015 at 21:18 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Gargamel-
I have merged your new nitroguanidine thread with another nitroguanidine thread.
If you please. If you don't have an actual lab you conducted to report on, or time to give the references /links to show your sources of information
leading to questions- and the topic has been repeatedly discussed, with multiple pre existing threads...POST YOUR QUESTION IN AN EXISTING RELATED
THREAD.
Thanks-
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | @ The_Davster,
Nitroguanidine, not guanidine nitrate!
From Explosives 4th revised and extended edition, Josef Köhler and Rudolf Meyer
p248 and 249
Nitroguanidine (H2N)2C=N-NO2
MW: 104.1 g/mol
Enthalpy of formation: -213.3 kcal/kg
OB: -30.7%
%N: 53.83%
Volume of detonation gases: 1075 L/kg
Heat of explosion (H2O gas): 687 kcal/kg
specific energy: 98 mt/kg
density: 1.71 g/cm³
melting point: 232°C = 450°F (decomposition without deflagration)
lead block test for 10g: 305 cm³
VOD at max density and confinement: 8200 m/s
impact sensitivity: up to 49 Nm --> no reaction
friction sensitivity: up to 353 N pistil load --> no reaction
critical diameter of steel sleeve test: 1 mm --> no reaction ...
Thus very insensitive even if confined!
Soluble in hot water but almost unsoluble in cold water. Sparingly soluble in alcohol and insoluble in ether.
Readily soluble in alkali (formation of salts NQ is a nitramine NH2-C(=NH)-NH-NO2).
Excellent chemical stability and not very sensitive to shock or impact.
|
From same book p 166:
Guanidine nitrate (H2N)2C=NH.HNO3
MW: 122.1 g/mol
Enthalpy of formation: -767 kcal/kg
OB: -26.2%
%N: 45.89%
Volume of detonation gases: 1098 L/kg
Heat of explosion (H2O gas): 482 kcal/kg
specific energy: 77 mt/kg
density: ??? g/cm³ (1.436 from Wikipedia)
melting point: 215°C = 419°F (deflagrate at 270°C = 518°F)
lead block test for 10g: 240 cm³
VOD at max density and confinement: ??? m/s
impact sensitivity: up to 50 Nm --> no reaction
friction sensitivity: up to 353 N pistil load --> no reaction
critical diameter of steel sleeve test: 2.5 mm
Thus very insensitive but more than NQ because it will blow a 25mm diameter pipe recipient with a 2.5 mm hole heated by Bunzen burners while NQ
doesn't with 1mm (Nitroglycerin does it at 24 mm)!
Soluble in alcohol and/or water.
--------------------------------------------------------------
Not asked but related to amino-guanidine nitrate...
From same book p379
Triaminoguanidine nitrate (H2N-NH-)2C=N-NH2.HNO3
MW: 167.1 g/mol
Enthalpy of formation: -67.07 kcal/kg
OB: -33.5%
%N: 58.68%
Volume of detonation gases: 1206 L/kg
Heat of explosion (H2O gas): 876 kcal/kg
specific energy: ?? mt/kg (must be >98 since VODG is bigger just as heat of explosion while energy of formation is less exothermic)
density: 1.5 g/cm³
melting point: 216°C = 420°F (decomposition-dflagrate at 227°C = 440°F)
lead block test for 10g: 350 cm³
VOD at 0.95 density and confinement: 5300 m/s
impact sensitivity: 4 Nm
friction sensitivity: over to 120 N pistil load --> crackling
critical diameter of steel sleeve test: ??? mm
Thus much more sensitive!
Prepared by reacting 1 mole of guanidine nitrate with 3 moles of hydrazine hydrate at 100°C for 4 hours.The reaction is accompanied by the liberation
of ammonia.
--------------------------------------------------------------
From Donald Haarmann (The Wizzards is In) VOD tables updated by Formatik in 2008
> Triaminoguanidine nitrate (TAGN)
VOD 5300 at 0.95
and 7930 at 1.46
LBT 350/117% vs TNT
Ref K&M and 2700 T28
> Guanidine nitrate (GN)
VOD 3700 at ??
LBT 10% vs TNT
LBT 110-140 thus vs TNT 36-46%
Ref 2700 G151 and Urbanski (2)
> Guanidine perchlorate
VOD 6000 at 1.15
VOD 7150 at 1.67
LBT 400 vs TNT 133%
Ref Urbanski (2) and Davis and 2700 G152
K&M= Köhler and Meyer
2700 = PATR 2700 Encyclopedia of Explosives and Explosive Items Vol.
Davis = Tenney L. Davis; The Chemistry and powder of Explosives (1943)
-----------------------------------------------------
Conclusions?
1°)Aminoguanidine nitrate must display properties between GN and TAGN.
2°)One can see that the enthalpy of formation of nitrate salt of amine is large, this comes mainly from the neutralization heat of the base by the
acid...most amine salts display such effect.
3°)The enthalpy of formation and heat of explosion goes up (less negative) by passing from GN to NQ (a kind of dehydratation)...this fact comes from
less water into the molecule...water in exhaust gases reduces the heat of explosion.
4°)The enthalpy of formation and heat of explosion goes up (less negative) by introducing hydrazine into the molecule see GN and TAGN; this fact
comes from endothermicity of hydrazine and that the N-N bond will form very stable N#N (dinitrogen) after burning releasing a lot of energy
5°)Perchlorates are better than nitrates, more dense salts results, better OB and energy output --> more sensitive and higher VOD or lead block
tests.
6°)Triaminoguanidine perchlorate must be a killer but also quite sensitive 

[Edited on 21-12-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Biguanide diperchlorate has VoD of 8490 m/s according to US4340755 A
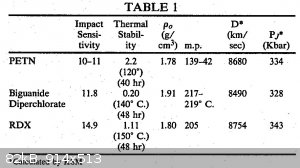
[Edited on 21-12-2015 by ecos]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Good Ecos!
Biguanide diperchlorate (BGDP) H2N-C(=NH)-NH-C(=NH)-NH2.2HClO4 is a kind of dimer of guanidine perchlorate (H2N-C(=NH)-NH2.HClO4)
H2N-(C(=NH)-NH)1-H .HClO4 (VOD 7150m/s at 1.67 g/cm³)
H2N-(C(=NH)-NH)2-H .2HClO4 (VOD 8490m/s at 1.91 g/cm³)
Now we need to find datas for:
-dinitro biguanide O2N-NH-C(=NH)-NH-C(=NH)-NH-NO2
-biguanide dinitrate H2N-C(=NH)-NH-C(=NH)-NH2 .2HNO3
-biguanide dinitroformiate H2N-C(=NH)-NH-C(=NH)-NH2 .2HC(NO2)3
-bisguanidine dinitrate H2N-C(=NH)-NH-NH-C(=NH)-NH2 . 2HNO3
-bisguanidine diperchlorate H2N-C(=NH)-NH-NH-C(=NH)-NH2 . 2HClO4 (probably stronger than BGDP)
-bisguanidine dinitroformiate H2N-C(=NH)-NH-NH-C(=NH)-NH2 . 2HC(NO2)3
-dinitro bisguanidine O2N-NH-C(=NH)-NH-NH-C(=NH)-NH-NO2
And azo dérivatives
H2N-C(=NH)-N=N-C(=NH)-NH2 . 2HNO3
H2N-C(=NH)-N=N-C(=NH)-NH2 . 2HClO4 (probably even stronger)
H2N-C(=NH)-N=N-C(=NH)-NH2 . 2HC(NO2)3
O2N-NH-C(=NH)-N=N-C(=NH)-NH-NO2
Formamidine dérivatives:
-formamidine nitrate H2N-CH=NH . HNO3
-formamidine perchlorate H2N-CH=NH . HClO4
-formamidine nitroformiate H2N-CH=NH . HC(NO2)3
-nitroformamidine O2N-NH-CH=NH
-aminoformamidine nitrate H2N-CH=N-NH2 . HNO3
-aminoformamidine perchlorate H2N-CH=N-NH2 . HClO4
-aminoformamidine nitroformiate H2N-CH=N-NH2 . HC(NO2)3
-nitroaminoformamidine O2N-NH-CH=N-NH2
-O2N-NH-CH=N-N=CH-NH-NO2
-diaminoformamidine dinitrate H2N-NH-CH=N-NH2 . 2 HNO3
-diaminoformamidine diperchlorate H2N-NH-CH=N-NH2. 2 HClO4
-diaminoformamidine dinitroformiate H2N-NH-CH=N-NH2 . 2 HC(NO2)3
Oxaladiamidine dérivatives:
-bisformamidine dinitrate H2N-C(=NH)-C(=NH)-NH2 . 2HNO3
-bisformamidine diperchlorate H2N-C(=NH)-C(=NH)-NH2 . 2 HClO4
-bisformamidine dinitroformiate H2N-C(=NH)-C(=NH)-NH2 . 2 HC(NO2)3
-bisnitroformamidine O2N-NH-C(=NH)-C(=NH)-NH-NO2
And
Diamino dérivatives of oxaladiamidine...
Tetramino dérivatives of oxaladiamidine...
Triguanide dérivatives H2N-(C(=NH)-NH)3-H
H2N-C(=NH)-NH-C(=NH)-NH-C(=NH)-NH2
--> dinitrate, diperchlorate, dinitroformiate, dinitro-
Thus stil a lot of simple compounds to discover/study with very potent explosive abilities probably over RDX, PETN...and maybe over HMX or CL-20
[Edited on 21-12-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Do anyone know any info for aminoguanidine nitrate ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Also related to aminoguanidine...diamino-tetrazine H2N-C(NN)2C-NH2
--> mononitrate, monoperchlorate, mononitroformiate and maybe dinitrate, diperchlorate, dinitroformiate.
Also dihydrazino-tetrazine H2N-NH-C(NN)2C-NH-NH2
--> mononitrate, monoperchlorate, mononitroformiate and maybe dinitrate, diperchlorate, dinitroformiate.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
-It has the formula H2N-C(=NH)-NH-NH2. HNO3
-It must display properties between those of guanidine nitrate and triamino guanidine nitrate...probably closer to TAGN.
--> d close to 1.5, VOD > 7500 m/s, Impact sensitivity in the range 3 < x < 10 Nm
Other infos
-Aminoguanidine can be made from equimolar cyanamide and hydrazine mix; and from guanidine with one hydrazine equivalent.
-Free aminoguanidine easily turns into diamino-tetrazine by air oxydation...
[Edited on 21-12-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
underground
National Hazard
   
Posts: 703
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by PHILOU Zrealone  |
-It has the formula H2N-C(=NH)-NH-NH2. HNO3
-It must display properties between those of guanidine nitrate and triamino guanidine nitrate...probably closer to TAGN.
--> d close to 1.5, VOD > 7500 m/s, Impact sensitivity in the range 3 < x < 10 Nm
Other infos
-Aminoguanidine can be made from equimolar cyanamide and hydrazine mix; and from guanidine with one hydrazine equivalent.
-Free aminoguanidine easily turns into diamino-tetrazine by air oxydation...
[Edited on 21-12-2015 by PHILOU Zrealone] |
I was thinking of making Aminoguanidine from nitroguanidine by reduction of nitroguanidine with zinc in acetic acid
http://www.sciencemadness.org/scipics/Engager/TzOTC/Syntheti...
Not the use of toxic hydrazine at all
diamino-tetrazine looks very interesting, what about diamino-tetrazine dinitrate ?
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Here's a thread I made a few years back which contains a ton of info on these guanidine compounds:
http://www.sciencemadness.org/talk/viewthread.php?tid=17908
|
|
|
Gargamel
Hazard to Others
  
Posts: 166
Registered: 9-3-2013
Member Is Offline
Mood: No Mood
|
|
Sorry Bert, I must have overlooked this thread.
I'm specifically after the practical usefulness of nitroguanidine.
The whole zoo of related compound that can be made with it is interesting, but of rather academical interest, because I cannot get the other precursor
materials in quantity.
Guanidine nitrate and sulphuric acid - OK, but not the rest.
By "Quantity" I mean amounts an amateur experimentor would work with, like 10g - 20g SCs.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Interesting, thank you for pointing this out!
So DougTheMapper and Praxichys are two faces of the same coin  , a kind of
schizophreny , a kind of
schizophreny  or simply loggin lost or the will to change? or simply loggin lost or the will to change?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Chisholm
Hazard to Self
 
Posts: 62
Registered: 2-4-2017
Member Is Offline
Mood: No Mood
|
|
So my vacuum pump has failed, and I'm having to do gravity filtration of the nitroguanidine in a Buchner funnel. This is an extremely slow process due
to the fine particle size.
I want to preserve the liquid, so I can boil it down to recover the sulfuric acid, but I'm concerned about the stability of nitroguanidine in acid. I
know that the concentrated solution from the addition of guanidine nitrate to sulfuric acid, if left to stand, eventually fails to produce
nitroguanidine upon dilution (CoPaE). But is NQ degraded in 25-20% sulfuric acid?
[Edited on 6-24-2017 by Chisholm]
|
|
|
| Pages:
1
2
3 |