| Pages:
1
2
3
4 |
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
but where is the fun in buying it? 
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
| Quote: | Originally posted by Picric-A
but where is the fun in buying it?  |
It is possible to make a few grams using a welder but it is quite laborious to make 50g.
I bought 500g for less than £10 including postage.
It is useful for making acetylene and acetylides.
A little goes a long way. A balloon filled with acetylene and air or 500mg of copper acetylide makes a spectacular bang.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
fair point, where did you buy it from, if you dont mind me asking?
Has anyone tryed what Berthelot did, HHeat it in a glass tube to produce Benzene and toluene?
i was thinkning a long test tube with the end in water or oil,
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I must be really boring.
I only use mine for its intended purpose, and although I understand the Chemistry and Mechanics behind it, to me they have a beauty all of their own
and still fascinate me.



\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
| Quote: | Originally posted by Picric-A
fair point, where did you buy it from, if you dont mind me asking?
Has anyone tryed what Berthelot did, HHeat it in a glass tube to produce Benzene and toluene?
i was thinkning a long test tube with the end in water or oil, |
I bought mine from an ebay trader that has ceased trading.
It is a question of looking around and having a bit of patience.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
Acetylene from Calcium carbide can be passed through a paste consistinf of mercuric sulphate and ammonium bisulfate to produce paracetaldehyde.
Paracetaldehyde is a convienent source of acetaldehyde for chemical experiments. Reference: Systematic Organic Chemistry. This book gives a
preparation of paracetaldehyde from acetylene.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
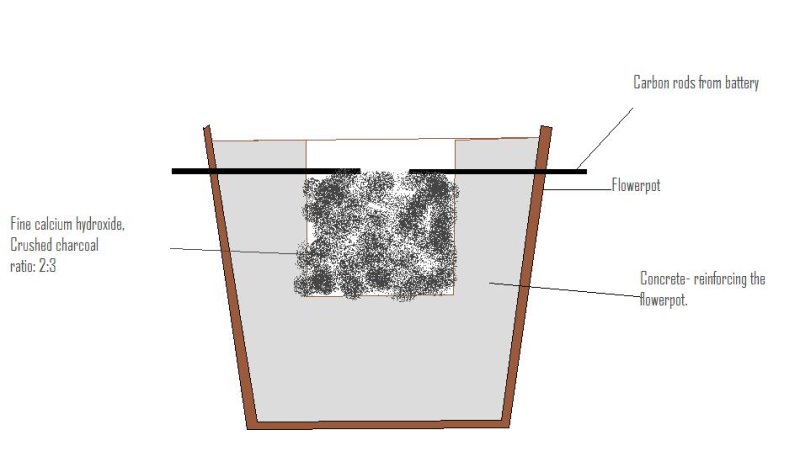
My attempt to make Calcium Carbide
Unfortunatly i dont have a decent camera, and so i will have to make do with paint 
I used my rather crappy arc furnace to do this. I took the carbon rods from a zinc chloride, D battery. at first when i cast te arc with them and
stopped it they burnt. I presumed this to be oil or something in the rods.
The mix was decent lab grade calcium hydroxide and crushed BBQ charcoal. the mix was fairly fine but not near airfloat constituancy (sp?).
The furnace was simply a flowerpot with heavy concrete reinforcements.
My power source was a 130amp arc welder although i used 80 amps.
When i cast the arc on the mixture it blew the mix everywhere, spraying a white power... i presumed this was CaOH. There was no grey blobs left, only
the white powder and i thought that the arc would radiate enough heat to reach the bottom of the pot but infact the mix on the bottom remained
untouched.... it was only the surface that turned white.
Upon adding some of this white powder to water, no bubbling was observed so this powder was infact CaOH.. 
Any ideas on how i could improve? maybe a change of Furnace?
Thanks,
Picric-A
[Edited on 9-1-2008 by Polverone]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Have read through this thread?:
http://sciencemadness.org/talk/viewthread.php?tid=2680&p...
Of special interest is the book by Stanfied that ignaro provides a link for called "The Electric Furnace."
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Yes i have read that thread several times,
Last time i did this procedure everything worked out well, i just dont know why i didnt work this time...
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Obviously the carbon is burning off the surface. You need to remove oxygen from the atmosphere and bury the electrodes in reaction stuff so it
doesn't all blow away. Gas is produced so granular, rather than powdered, reactants is a plus!
Tim
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Cool thanks for the help guys, when i get round to making a new furnace and doing the reaction again i will tell you how it goes!
Picric-A
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Magpie
Of special interest is the book by Stanfied that ignaro provides a link for called "The Electric Furnace." |
archive.org has a DJVU version of that up now:The Electic Furnange. Yes, that's some bad, bad OCR happening.
|
|
|
tapira1
Hazard to Others
  
Posts: 168
Registered: 9-10-2006
Location: Here!!!
Member Is Offline
Mood: 
|
|
Drying EtOH
Ethanol is best dried employing magnesium, via the magnesium ethoxide. Magnesium can be obtained from pen sharpeners or magnesium car wheels.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
I wonder if CaC2 could be made by electrolysis of fused CaCl2 or CaCl2/CaO with a graphite or other carbon cathode. I intend to find out as soon as I
get my equipment ready.
The mind cannot decide the truth; it can only find the truth.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I guess by this method you are hoping the Calcium metal produced would react with the Carbon cathode.
Unfortunatly this method requires around 900 degrees, if not more, so it is unlikly you would get any.
Also how would you seperate the CaCl2 from the CaC2?
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
Well it is quite normal for such a cell to run at 900°C or more, and I do not believe CaC2 would be soluble in CaCl2. It beats the heck out of the
2000°C required for the traditional method. Quite frankly I do not see why the FFC Cambridge Process extractions of boron and silicon would not form
the respective calcium boride or silicide similarly to this idea; if allowed to run until the oxide was depleted, it no doubt would, or perhaps it
does anyway.
Sadly I was not able to experiment tonight on the matter. My steel cell crucible sprung a small leak along the welds, and I got a lot of fused CaCl2
poured out into the bottom my furnace, causing major problems. I cannot re-melt it and get it out because the bottom area of the furnace does not get
that hot. The molten salt was quite solible with the furnace refractory as well so it is bonded. Just keeping the furnace from being destroyed now
by atmospheric water absorption will be a huge challenge. It's a heck of a mess.
Dumping sodium carbonate in to react up the CaCl2 as it goes into solution would seem the best course of action at this point. It's a heck of a mess.
Dumping sodium carbonate in to react up the CaCl2 as it goes into solution would seem the best course of action at this point.
The mind cannot decide the truth; it can only find the truth.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I agree CaC2 wouldnt be soluble in it but are you just going to scrape it out as it is formed?
I think it is more trouble than its worth, you might aswell take the calcium produced and heat that in a crucicible with Carbon...
But i still think an arc furnace is an easier method, i produced around a Kilogram, if not more, from my simple concrete lined arc furnace, powederd
by my arc welder, with CaCO3 from the local chalk mine and Charcoal made on the bonfire, simple and cheap.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
Extracting the actual calcium metal from an electrolytic cell like that is easier said than done. Calcium is soluble enough in the melt (3.9% molar)
that there are problems depositing it on a fixed cathode. It is much easier to take advantage of its reactivity right at the cathode under the
protection of the salt bath and the heat that is already there. I am thinking now that it may be more suitable to use a steel cathode surrounded by
loose coal, charcoal, or coke, because the calcium would have trouble diffusing into a dense graphite cathode.
When the cathode racks are pulled from the melt, they would be covered with electrolyte, and the broken up material could then be washed with alcohol
or something (as long as it doesn't react with the carbide) to leech out CaCl2 and leave the carbide. If the carbide was just used for generating
acetylene, the CaCl2 would not even be a problem if it was all broken up together.
Anyways I am just trying to think of more efficient methods (from an energy standpoint) than the incredibly hot arc furnace. It may be more
complicated at a small scale, but beyond mere kilograms it could be economical. I am always interested in exploring the various uses of the
technology of fused CaCl2/CaO electrolysis.
[Edited on 28-11-2008 by kilowatt]
The mind cannot decide the truth; it can only find the truth.
|
|
|
Nevermore
Hazard to Others
  
Posts: 140
Registered: 3-5-2003
Location: China at the moment
Member Is Offline
Mood: shopping businessman
|
|
| Quote: | Originally posted by kazaa81
Hallo to all,
I'm looking for CaC2 in Italy, but I haven't find anything. Is it today used?
I don't know, because acetylene lamps aren't used today.
Where and under what name I can buy it (except scientifical suppliers)?
Thanx for help.
[Edited on 3-9-2004 by chemoleo] |
Calcium carbide is sold commonly in Italy.
Try looking at the shops that sell equipment for fishing (look for "carburo").
Near the marina or near any fisherman place you will find plenty of it, and cheap, around 6-7€ per Kg.
Nevermore!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
I just buy it , but here is simple experimental prospect of easily making it worth a try.
http://www.sciencemadness.org/talk/viewthread.php?tid=13144#...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Here I thought the only way to obtain Aluminum other than reduction of
it's Chlorides with alkali metals was by the Hall electrolysis of bauxite in a
cryolite melt. Alumina Al2O3 can be reduced by acetylides and if it is mixed
with carbon and an amount of catalytic Calcium oxide CaO to first produce
CaC2 , that in turn reduces the Al2O3 yielding Aluminum ! How cool is that !
|
|
|
metalresearcher
National Hazard
   
Posts: 757
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Nice to read all about making CaC2 !
I made CaC2 in an arc furnace using a 150A hardware-shop-at-the-corner welder and crushed marble with charcoal.
When dropping pieces into water it bubbled and the gas, presumably C2H2, burned with a sooting flame.
More info how I did it on my site: www.metallab.net/arcmelt
|
|
|
doofos
Harmless

Posts: 2
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Friction heater if you don't want to use electricity.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
A few points. First per Wikipedia (http://en.wikipedia.org/wiki/Acetylide ), to quote:
"Some metal acetylides are traditionally called carbides. For example, lithium carbide and calcium carbide are really derivatives of C2 2−."
Also: "Acetylides of the alkali metals can be prepared by deprotonation of acetylene in liquid ammonia."
In fact, per this source, "A treatise on chemistry, Volume 2, by Henry Enfield Roscoe, Carl Schorlemmer, page 560. link: http://books.google.com/books?id=030GAQAAIAAJ&pg=PA560&a... to quote:
"It [referring to CaC2] is best prepared pure by passing acetylene into liquid ammonia containing metallic calcium [2] and heating the resulting
compound, CaC2,C2H2,4NH3, or by the action of acetylene on calcium hydride, the compound CaC2,C2H2, being first formed.[3]"
Interestingly,this manner of preparation of CaC2 closely parallels that for Na2C2 for which it is structurally related. To quote Wikipedia (link http://en.wikipedia.org/wiki/Sodium_carbide ):
"Acetylides
Several carbides are assumed to be salts of the acetylide anion C2 2– (also called percarbide), which has a triple bond between the two carbon
atoms. Alkali metals, alkaline earth metals, and lanthanoid metals form acetylides, e.g., sodium carbide Na2C2, calcium carbide CaC2, and LaC2.[1]"
Here is a cited preparation for Na2C2 (see http://sodium.atomistry.com/sodium_carbide.html ), to quote:
"When acetylene reacts with sodium, either in the metallic state, or as hydride, or in solution in liquid ammonia, a substance of the formula
C2Na2,C2H2 is produced. When heated in vacuum, or in a current of hydrogen at 220° C., it evolves acetylene, leaving sodium carbide. The carbide is
very reactive, readily undergoing decomposition with deposition of carbon, and decomposing water with evolution of acetylene."
This source ( see http://books.google.com/books?id=YyoUNYgBYJoC&pg=SA1-PA1... ) notes that when Ca is dissolved in liquid ammonia, a paramagnetic, highly
conducting, highly reducing deep blue liquid is formed, these properties being attributed to the presence of solvated electrons in solution,
[Edited on 4-8-2013 by AJKOER]
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
I knocked up a quick video on making Calcium carbide vie electrolysis this afternoon, it's not great quality but you can get the idea https://www.youtube.com/watch?v=xSAziTIHs3w
|
|
|
| Pages:
1
2
3
4 |