| Pages:
1
2
3
4
5
..
18 |
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I would suspect the NaOH would dissolve the TiO2 layer and form sodiumtitanate, but I may be wrong...
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
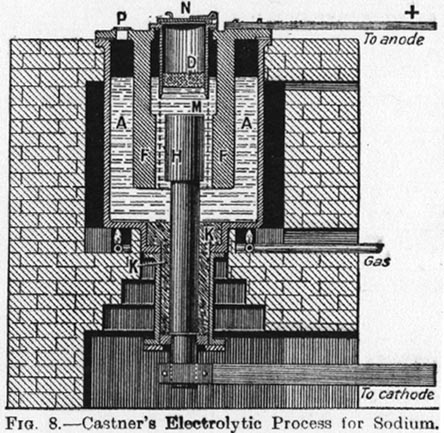
Like I said, a picture of another, Castner Tiegel. This one with additional explantions.
| Quote: |
The sodium hydroxide, contained in an iron pot set in brickwork, is melted by means of a ring of gas jets placed underneath; and kept about 20 C above
the m.p. (318C) of sodium hydroxide. The cathode, H, of nickel or iron rises through the bottom of the iron pot, A,
Fig. 8, and is maintained in position by a cake, K, of solid sodium hydroxide in the lower part of the pot. THe anodes,
F, several in number, are suspended around the cathodes from above. A cylindrical vessel, ND, floats in the fused
alkali above the cathode, and the sodium and hydrogen liberated at the cathode collect under this cylinder. The hydrogen escapes through the cover
and the atm. of hydrogen in the cylinder protects the sodium from oxidation. A cage of nickel-wire gauze, M, seperates the anode,
F, from the cathode, H. From time to time the sodium D, is skimmed off by means of a perforated
ladle, which retains the liquid metal, but allows the molten hydroxide to flow back. The oxygen liberated at the anode escape via the vent
P.
|
Simply a beautiful process. One day it will be mine. As for vulture's comment on the use of titnium I didn't even think of that, I was
more worried about hydrogen embrittlement, titanium is strikingly succeptable to that.
Also, I found "Sodium chloride mixed with powdered lead heated red hot in a closed retort gives metallic sodium:
2NaCl + Pb -----> PbCl2 + 2Na
I guess I would list that just for completeness seeing as how our current research into aluminum is paying off so well.
Also, while I'm on a roll, "Upon heating a molten mixture of sodium carbonate and sodium cyanide at a temperature of 1000C, metallic sodium,
carbon monoxide, and nitrogen are formed."
2NaCN + Na2CO3 ----> 4Na + 3CO + N2
[R. Franchot, Ind. Eng. Chem., 16, 235 (1924)]
I also found references to sodium carbonate being reduced by both magnesium and aluminum turnings to yeild sodium metal, which I believe has been
hinted at if not outright stated already.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Bromic,
I'm not convinced the aluminium method is worth following up, for reasons Ive explained.
vulture,
I concurr with the worry about titanium in molten hydroxide.
Organikum,
Then there is a problem, becuase if the bell isnt electrically connected to the copper nail at the start of the reaction, it soon will be. A cell
where both electrodes enter from the top seems to require a molten hydroxide stable insulator just like the other designs, and I havnt been able to
find one I can use. The one good suggestion that was made to me was sintered magnesia containing 1% or so waterglass, which only reacts with the
hydroxide very slowly, but I lack a suitable furnace to make it in.
Any idea what insulators the Castner Tiegel cell used?
It would also be nice to know how big the holes in the ladle need to be for surface tention to retain the sodium, but allow the hydroxide to pass
through.
If the battery is just for smoothing the current, then it is not useful in this application. If the cell has 12v over it then too much power is being
wasted for the current that is flowing and it needs redesigning.
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
Look on the first diagram on the first page, The Holzring is the anode insulator, its made of wood.
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Marvin
| Quote: |
Then there is a problem, becuase if the bell isnt electrically connected to the copper nail at the start of the reaction, it soon will be. A cell
where both electrodes enter from the top seems to require a molten hydroxide stable insulator just like the other designs, and I havnt been able to
find one I can use. The one good suggestion that was made to me was sintered magnesia containing 1% or so waterglass, which only reacts with the
hydroxide very slowly, but I lack a suitable furnace to make it in.
|
No really not. Hole in bell with filed down nail through it fixed by ovencement as no molten Na should reach the holes for hydrogen escape would be
blocked and boom it makes.
| Quote: |
It would also be nice to know how big the holes in the ladle need to be for surface tention to retain the sodium, but allow the hydroxide to pass
through.
|
Eh? surface tension? I guess this is an misunderstanding. Hydroxide passing through what? Why?
Refill: Take plier. Open lid. Throw in NaOH. Close lid. Do fast.
for the rest:
no. no. no. no.
current efficiency in a quick and dirty tomatosoupcansetup?
no. no. no.
No. sorry.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Hermes, there seems to be something inbetween the solidified melt and the holtzring. Its just labled isolation in what I can see, but it must be
molten sodium hydroxide stable and an insulator.
Wood is attacked by molten sodium hydroxide and starts to decompose over about 270C anyway. I dont think we can use this.
Organikum,
From the description it seems the molten sodium will short circuit the nail and the bell long before the bell has filled with sodium. I dont
understand how this is prevented.
One of the features of the castner process is that the sodium is removed by a perforated ladle that retains sodium while draining hydroxide. It would
be nice to know what hole sizes are needed for this.
Adding a battery is only useful over a narrow range of cell voltages. If the cell is not designed to work over this range, the battery will not help.
If it is designed to work over this range then its been designed specifically to work at a voltage that wastes power. Its a lose-lose situation.
Bromic acid,
Very nice going. If you are willing to swap magnesium for sodium potassium etc, and I would be if I could get magnesium cheaply, then you might
prefer the organic solvent method in the patent. Decent yeilds and its easier to get the metal as a single ingot relativly free of magnesium
oxide/hydroxide.
Aluminium and sodium hydroxide is supposed to go mainly to Na3AlO3, a mixture that was formerly used to defrost oil wells and which produces no sodium
directly, formation of sodium is a side reaction with the coproduction of sodium metaaluminate. Magnesium seems the only feasable metalic reducing
agent which limits most peoples ability to use this.
Kaboom, now that would be hydrobromic acid wouldnt it 
[Edited on 15-12-2003 by Marvin]
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
molten sodium
seems to be a bad conductor for electricity, Marvin.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Molten sodium is one of the best liquid conductors.
Solid copper around e-8, liquid sodium around e-7 liquid mercury about e-6 (perohmpermeter). Its undoutably much better than the molten salt its
displacing/floating on.
Theoretic, based on what?
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
outch.
but it works nevertheless, also I never thought on this ??
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Calcium
I know this thread is about making sodium, but calcium is a similar metal. I found out that CaCl2 actually has a slightly lower melting point than Ca
does. Calcium also does not burn nearly as readily as sodium does (in fact, I had a hard time getting a small hunk of Ca to burn even with a
blowtorch). Yet it still reacts fairly well with water. And CaCl2 is cheap and plentiful (dehumidifier powder or ice melter).
I would think Ca would be significantly easier to make than Na. Might be a good first try for someone who has never made Na.
I tried making sodium from lye when I was 14. I heated up some in a large spoon, using the spoon for the anode and a wire for the cathode. As I
recall, I got vigerous bubbling (much more so than when electrolysing water, for example). The hydrogen kept exploding and blowing out the alcohol
lamp I was using to heat it. I had a small gray lump near the cathode that I tried putting some into water with a tweezers. Nothing appeared to
happen. But when I touched the lump again with the wet tweezers there was an orange spark. Have never tried making it again since then.
Hodges
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Yes, calcium would be easier to make than sodium.
I remember reading a description of the setup to make calcium in the lab. It involved the use of a screw-like iron electrode which would be lifted
from the molten CaCl2 as calcium was being deposited, leaving only a tip of calcium in.
I'm traveling now and can't read my books to find out why can't you just leave the deposited calcium in the molten salt. This would
even prevent oxidation.
When I return home, I will find out and post. Maybe we should start a "Calcium!"
thread.
Although you can use calcium to dry solvents and make some interesting reactions, sodium BURNS, or even EXPLODES with water! UNDER water! You
can't beat that! A "Calcium!" thread sure would not be as popular as this one.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
A bit off topic but how much shouldc a graphite electrode cost?, I saw one today for 12$ CDN. It is about 6mm wide by 15 cm long
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Wow, thats really expensive. I got a pack of 6 of carbon rods about 1 cm x 15 cm for less than $2.
Edit:
I dont live in the US, so you wouldnt know were I got them from anyways 
But if you insist, I got them at a local shop that sells plants stuff.
[Edited on 8-1-2004 by Saerynide]
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Get it while it's hot!
http://members.aol.com/bromicacid/sodium/index.htm
I don't know how long I'll leave this up, maybe someone can convert to .pdf and host it but for now here it is, go to the page and gander at
the scans I made from "Sodium: Its manufacure, properties, and uses" It is the best reference Ive come across yet regarding both chemical
and an overview of the electrochemical methods to this wonderous element. I have the whole chapter on the production of sodium metal scanned in.
Like I said, get it fast, download it, whatever!
[Plus when I take it down I have to delete this post so that my last post is not a lie.....]
[Edited on 1/13/2004 by BromicAcid]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Ahhh, I remember reading that book some months ago. BromicAcid, do you have higher-resolution scans on your computer, that OCR would work on? Whether
you do or not, I'll gladly host a PDF made from the scans (someone else will need to assemble the PDF as I don't have access to Acrobat at
the moment).
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
On the topic of separation of sodium from carbon that fell off the electrode... ...sodium forms sodium carbide with carbon, separation must be quick.
An easily meltable salt could be used to do that, it would sink to the bottom, and carbon would sink to the bottom of it, while sodium would float on
top. Also sodium carbide would dissolve in the molten salt.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
sodium production chapter converted
BromicAcid: the images you had on your website have been turned into a PDF and are now in the <A
HREF="http://www.sciencemadness.org/library/">library</A>. If there are other materials that you want to scan and put online in the
future, I suggest scanning in black and white at 300 or 600 DPI (or scanning in grayscale and then converting to black and white) since it's
cleaner, compresses down to a smaller size, and looks better on screen/in print. But thank you for making that chapter available the way you did.
PGP Key and corresponding e-mail address
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
Polverone:<blockquote>quote:<hr>BromicAcid: the images you had on your website have been turned into a PDF and are now in the
library<hr></blockquote>I downloaded it. it gives this error when I open it:
<i>There was an error opening this document. The file is damaged and could not be repaired.</i>
(Thank you in advance)
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
you are quite right
The file I had locally was twice the size of the one in the library. I guess the upload got interrupted before. I have verified that the file now in
the library can be downloaded and viewed.
PGP Key and corresponding e-mail address
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Castner Tiegel remark
I was irritated all time because of Marvins remark that the molten sodium would give a shortening of the electric current between the "bell"
and the central electrode and make the design unworkable.
But I had done it and it worked.
How could this be?
Solution: The iron bell works is cooled by the air. At the edges inserted in the molten NaOH the NaOH solidifies and forms such an isolator on the
bell. This works because the temperatur of the NaOH is only slightly above the melting temperature of NaOH and the superior heattransfer properties of
iron compared to NaOH.
Try it!
Just put something from iron into just molten NaOH and you will see the solidified NaOH forming a stable protection layer on the iron. And this layer
wont dissolve also not after times.
Nothing shortens here.
Hope this answers your question Marvin?
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Electrolysis of Sodium Hydroxide

- Picture shows my nickel crucible with some sodium hydroxide pellets in it. Anode is just the crucible and the cathode that decends into it is
nickel as well. Below it is the torch that I was using to heat it.
- Too awhile for everything to melt. The hydroxide pellets on the top solidified together and formed a crust, I didn't realize it was all
liquid underneath till I poked at it with a nickel rod, it all caved in and melted together. The solution was white till it all dissolved then turned
crystal clear. Electrolysis was begun using a 9V battery.
- Very soon after this there were bubbles at both the edge of the crucible and at the cathode. No sodium though, but the solution darkened to a
yellow color.
- Thinking that maybe the solution was too hot and that any sodium being produced was reacting back with the hydroxide at this temperature I
turned off the heat. A crust quickly formed over the top that bubled up due to gas formation. Some sodium may have been formed but I could not tell.
After awhile the bubbles stopped so I turned the heat back on.
- Not long thereafter I decided that maybe it wasn't a good idea to use the crucible as the anode so I grabbed a piece of nickel rod and put
the clip on that and manually held it in place. Bubbles started to rise immediately but the hydroxide solidified around it and it took awile to take
on enough heat to where it conducted again. You can see a black crud around it, I believe the sodium was going into solution and where ever the
hydroxide was solidifying it was darkening more and more.
- The hydroxide solution turned almost black and no longer produced bubbles. The metaloid that has been mentioned. I discontinued electrolysis
and heating. I let it cool a bit and added water, very violent reaction but it was a block of hot hydroxide after all.
During the whole procedure I saw no sodium gobules. But as the solution darkened I was sure there was some around there somewhere. The nickel
crucible came out with no noticeable errosion but it had a black residue on it. What's everyone think, was the NaOH too hot, too much voltage,
not enough, current, electrolysis really isn't my strong point. Also this cell really isn't anything like the one that I've been
working on, this was mostly just a proof of principle thing makes it even worse that it didn't work for me.
[Edited on 4/4/2004 by BromicAcid]
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
I think if by "9V battery" you mean one of those tiny things a few centimeters square and about 1 centimeter thick, it did not have enough
capacity to do much electrolysis. A power supply is best. Lacking that, use a 12-V auto or motorcycle battery. Lacking that, use a 6V alkaline
lantern battery.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I have also tried NaOH electrolysis a number of times (under conditions as you describe), with the same results as you. A blackish residue was left,
that violently reacted with water.
I used a powersupply, with as much amperage as it could take (the amperage being restricted by the resistance), @ 12 V.
Never any success that way. I believe it can't be done this way, this has to be done in a properly designed vessel, with proper temp. control,
etc.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I had several sucesses with small amounts of hydroxide and one failed attempt when I tried to scale it up (before I had a good idea how.
Hodges is right, you arnt getting enough current from the battery. I used a 12v PSU capable of delivering about 7A. The voltage was much higher than
it needed to be, the PSU sounded almost like it was directly shorted when running. 7A though is the very minimum Id consider using as a proof of
concept. A car battery or motorcycle battery might deliver far too much cuttent for too short a time to be useful. Formation of sodium was very slow
at 7A though its halo was impressive, best part of the experiment IMHO.
Ideally you should be pumping almost almost enough power into the cell that it stays molten without external heating - this makes it much easier to
keep it around its melting point. I would also expect an induction period before sodium starts to form as the mixture dehydrates.
Edit,
Also watch out for the small explosions as the sodium sets the hydrogen bubbles off. This tends to send sodium metal flying round the room and
limited the amount I could let form as a globule before I had to remove it, or lose it. Eye protection a must, though I think you know this. Thick
gloves also as the fumes tend to attack skin.
[Edited on 5-4-2004 by Marvin]
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by hodges
I think if by "9V battery" you mean one of those tiny things a few centimeters square and about 1 centimeter thick, it did not have enough
capacity to do much electrolysis. A power supply is best. Lacking that, use a 12-V auto or motorcycle battery. Lacking that, use a 6V alkaline
lantern battery. |
I agree. You need much more current than this batteries can provide. At least a couple of amperes (edit) considering the size of your setup.
When I tried to do an open air electrolysis of NaOH, I could not get any metal due to the fact that sodium burns at this temperature, but I could see
the little orange sparks in the cathode. Can you see those?
You seem well equiped, can't you built the "bell" proposed some time ago in this thread?
[Edited on 5-4-2004 by Tacho]
|
|
|
| Pages:
1
2
3
4
5
..
18 |