| Pages:
1
2
3
4
5
..
23 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Polynitrogen moieties
A concise and informative overview of the current state of the art
of energetic molecular compounds can be viewed at this site here _
http://enermat.org.ru
click the navigational links < back || next > on the bottom of each
page or else their url's in order here _
http://enermat.org.ru/pyrazines.html
http://enermat.org.ru/furazans.html
http://enermat.org.ru/tnaz.html
http://enermat.org.ru/nitro.html
http://enermat.org.ru/ammomium.html
Just a thought
It would be interesting to see if compound number 6 depicted at the botom
of this page => http://enermat.org.ru/pyrazines.html
could add two azide groups by the action of N2O on the two amines. What
interesting heterocyclic variant may isomerize from that ?
______________________________________________
Theoretical analysis of polynitrogen moieties
http://www.qtp.ufl.edu/~bartlett/pdf/polynitrogen.pdf
Abstract:
Provides a theoretical analysis of fanciful hypothetical energetic Nitrogen moieties.
I have an idea that the eight member ring structure depicted on page 52 above
can be acheived , at least as a side group. Take away one carbonyl from cyanuric acid
and you have another simpler organic acid , 1H-1,2,4-Triazol-3,5-diol ( Urazol ) below left
http://www.emolecules.com/cgi-bin/more?vid=595279
which much as TCCA does , also tautomerizes into another pH dependent form
1H-1,2,4-Triazole-3,5-dione ( Urazole ) below right
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=7291...
 [img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=72916[/img] [img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=72916[/img]
More references _
http://www.emolecules.com/cgi-bin/more?vid=595279
http://dtp.nci.nih.gov/dtpstandard/servlet/ChemNameServlet?t...
http://www.ncbi.nlm.nih.gov/sites/entrez?term=3232-84-6%7E%5...
Properties _
http://www.acros.com/DesktopModules/Acros_Search_Results/Acr...
http://iaspub.epa.gov/srs/srs_proc_qry.navigate?P_SUB_ID=113...
The following link in beteween < > pointy brackets will not parse correctly
copy and paste it into the address bar of your browser
< http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+toxline:@term+@rn+3232-84-6+@OR+@all+"" >
Equilibrium acidity constants have been determined for 1,2,4-triazolidine-3,5-dione (urazole),
several substituted urazoles, and other related acids, in both dimethyl sulfoxide (DMSO) and
aqueous solution. In DMSO, urazole has a pKa of 13.1. In water, urazole has a pKa of 13.1. In
water, urazole has a pKa of 5.8. In general, N-methyl and N-phenyl substituents are found to
acidify the urazole moiety, in both DMSO and water. The acidifying effects of these substituents
are attenuated by a factor of 3.3 in water. The solvent effects are ascribed to the aqueous
stabilization of urazole anions via hydrogen-bonding interactions and the aqueous-promoted
relief of lone pair-lone pair electronic interactions that manifest themselves upon deprotonation
of a hydrazyl proton in 1 and related species. That a hydrazyl proton in 1 is at least as acidic as
the imide proton in 1 is confirmed by comparison of 13C NMR spectra for the urazoles and
related nitrogen acids with 13C spectra for
- - - - - - - - - - - - - - - - - - - - - - - -
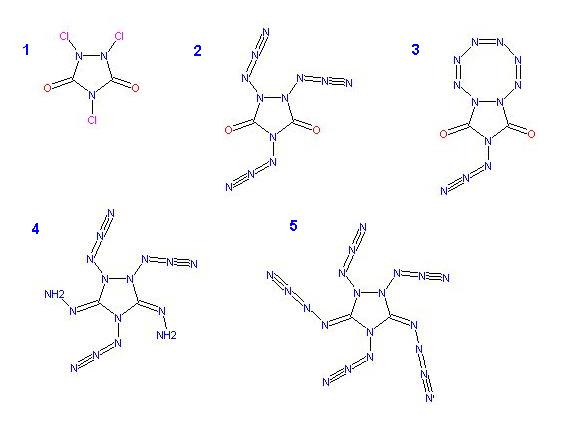
N-Chlorination of the amines to obtain 1,2,4-Trichloro-1,2,4-Triazole-3,5-dione _ 1 )
and reacting this with NaN3 should yield , 1,2,4-Triazido-1,2,4-Triazole-3,5-dione _ 2 )
essentially a symetric di-azido-hydrazyl group
which could isomerize producing an octagonal nitrogen ring _ 3 )
A much more fanciful notion would be to further substitute the Urazole carbonyls with hydrazine
C:O + H2NNH2 => H2O + C:NNH2 , to form Hydrazone groups _ 4 )
these can be reacted with nitrous oxide N2O
C:NNH2 + N2O => H2O + C:N-N=NN to obtain N4 Azidamines _ 5 )
( related thread here _ http://www.sciencemadness.org/talk/viewthread.php?tid=9370 )
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Are there examples in the literature of carbonyls next to N-azides? Just wondering because surely the carbonyl will destablise any azide moiety due to
its electron-withdrawing effect?
Beautiful summary in the enermat.org site... almost worth saving it and uploading it here!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
Are there examples in the literature of carbonyls next to N-azides? |
literature ? what literature  , no none that I know of , bear , no none that I know of , bear
in mind I'm not a chemist so if it's not feasible then that's that.
| Quote: | Originally posted by chemoleo
Beautiful summary in the enermat.org site... almost worth saving it and uploading it here! |
U P D A T E
New Energetic Materials Is available as a pdf here _
http://my-lair.narod.ru/New_Energetic.pdf
* N O T E *
You must copy this url and paste it into the address bar of your browser to download
_________________________________
Way ahead of you , I already had , good notion to post it , only 1/2 MB
unfortunatley I know of no way to do this in the forum server itself
using the zip format ( problems unpacking )
For those who like me their unzipping utilities are " no good "
here is are three tested outside hosts , download and save
before opening _
http://www.keepmyfile.com/download/f364832074487
http://www.badongo.com/file/7077815
http://mihd.net/vp6tjn
If you wan to try to open the file attached below , try this
1. download and save to your computer
2. delete the - .zip - extension
3. click the file and select the utility to open it from the popup dialog box
4. drag the file you see inside to the desktop and click that to open it
5. again, repeat step 3
6. you should now see the contained .mht files and be able
to extract or view them directly in your browser.
[Edited on 11-1-2008 by franklyn]
Attachment: New Energetic Materials.zip (530kB)
This file has been downloaded 2301 times
|
|
|
Axt
National Hazard
   
Posts: 856
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The majority of that site, up the the plasticisers is directly copied from Thermochimica Acta 384 (2002) 187–204. I cant find the article for the
plasticiser ect. portion but it has been available as pdf at some time here.
Attachment: a review of energetic materials synthesis.pdf (278kB)
This file has been downloaded 5219 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Energetic high Nitrogen Heterocyclic compounds
| Quote: | @ Axt
Funny I had that since early September
didn't make the connection though |
US patents of interest with preparations
7119179 - Prep of high Nitrogen compounds ( many types )
6657059 - 3,6bis(1H-1,2,3,4-Tetrazol-5-ylamino)-1,2,4,5-Tetrazine
6570022 - bis-(1(2)H-Tetrazol-5-yl)- Amine
6552201 - 3,3'-Diamino-4,4'-Azo Furazan
6458227 - 3,6-bis(1H-1,2,3,4-Tetrazol-5-ylamino)-1,2,4,5-Tetrazine
6388087 - 3,3'-Dinitro-4,4'-Hydrazo Furazan
6358339 - 3,3'-Diamino-4,4'-Azoxy Furazan
6342589 - 3,3'-Azo(bis)(6-Amino-1,2,4,5-Tetrazine)
6312537 - Tetrazoles
U P D A T E
Paper with preparations of insesitive aza-heterocyclic nitramine ureas
http://my-lair.narod.ru/v26pp63-68.pdf
* N O T E *
You must copy this url and paste it into the address bar of your browser to download
[Edited on 11-1-2008 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
TNPDU
Edit option lapsed so item of dead link above is now available here
http://www.sciencemadness.org/talk/viewthread.php?tid=7518&a...
Synthesis, Characterization and Thermal Behaviour of
(TNPDU) 2,4,6,8-Tetranitro-2,4,6,8-Tetraazabicyclo[3.3.1]Nonane-3,7-Dione
and one of its Methylene Analogues
A. K. Sikder, G. M. Bhokare, D. B. Sarwade, J. P. Agrawal
Propellants, Explosives, Pyrotechnics 26, 63 - 68 (2001)
On N-Cl substitutions
http://www.sciencemadness.org/talk/viewthread.php?tid=4282&a...
.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by chemoleo
Anyway, this article also mentions dinitrobiuret, which was commonly used to assay protein concentrations (i.e. protein + CuSO4 + NaOH --> deep
blue colour).
It is
H2N-CO-NH-CO-NH2
so presumably the NO2 groups are on the NH2's which gives
O2N-HN-CO-NH-CO-NH-NO2
I seem to remember that biuret is made by the condensation of urea, that is heating it up to its decomposition point. I searched, couldnt find info on
it though - at least in my books. But whether that works with nitrourea is definitely a different issue.
Any thoughts?
|
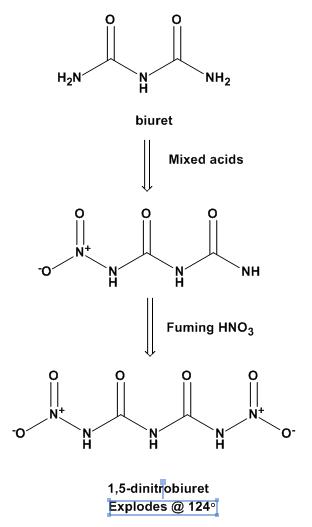
You have the structure correct. It is 1,5-dinitrobiuret, aka DNB.
There is not much literature on this, which is surprising when you consider thae fact that it was first made in Germany in 1898! It was resynthesized
in Germany in 2004.
Heating nitrourea to decomposition just gives you NOx. They use a 2-step nitration of biuret: first with mixed acids to get 1-nitrobiuret, then with
fuming HNO3 at ice temperatures to get the DNB.
The Thiele prep is attached.
[Edited on 2-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Here is the original article abstract.

Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
"nitrobiuret ... prepared by adding biuret in small portions to a mixture of concentrated nitric and sulphuric acids, separates from water as a white,
crystalline powder"
100g biuret is added under stirring into an ice cooled mixture of HNO3 (66 ccm, D = 1.4) and conc. H2SO4 (250 ccm), after the end of the reaction (2
hours) it is poured onto ice, and the nitrobiuret is washed with water and alcohol. The remaining nitrobiuret in the mother liquor is obtained by
precipitating with mercury nitrate.
"produced when the finely divided nitro-deriative is added to fuming nitric acid cooled with a freezing mixture".... The solution is then evaporated
in a vacuum in the dark over "Natronkalk" (Ca(OH)2 plus NaOH), and H2SO4. The nitric acid is 100% pure.
The German references say it just deflagrates at 124 deg. The potassium salt (K2C2HO6N5) also deflagrates when heated.
[Edited on 2-8-2008 by Schockwave]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
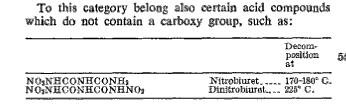
If DNB is such great stuff, why isn't there a lot of literature on it? There are only half a dozen U.S. patents that mention its use in coatings &
a 1960 patent with a brief mention of its use in a diazo reprographic system. Note the different decomposition temperature given.
[Edited on 2-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Ritter
If DNB is such great stuff, why isn't there a lot of literature on it? There are only half a dozen U.S. patents that mention its use in coatings &
a 1960 patent with a brief mention of its use in a diazo reprographic system. |
The material has been rediscovered, interest may grow. A blast from the past. Back from the dead. If the lower decomposition temperature is correct,
then it has for the purposes of commercial application the draw back of a low decomposition point. Another thing is that it forms salts, so may be
incompatible with metals. Despite those it has good chemical stability, and is up there with the greats PETN, HMX, RDX, etc. in terms of energy
output.
| Quote: | | Note the different decomposition temperature given. |
Whatever its decomposition temperature really is, I usually tend to take patent values secondary to periodicals. But at least we know the range. That
is at least until someone else whips out another figure.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Next-generation Explosives: More Power And Safety Without The Pollution
ScienceDaily (May 27, 2008) — Scientists in Germany are reporting development of a new generation of explosives that is more powerful than TNT and
other existing explosives, less apt to detonate accidentally, and produce fewer toxic byproducts.
Their study of these more environmentally friendly explosives is scheduled for the June 24 issue of ACS’ Chemistry of Materials, a bi-weekly
journal.
In the new study, Thomas M. Klapötke and Carles Miró Sabate point out that conventional explosives such as TNT, RDX and HMX, widely-used in military
weapons, are rich in carbon and tend to produce toxic gases upon ignition.
In addition to polluting the environment, these materials are also highly sensitive to physical shock, such as hard impacts and electric sparks,
making their handling extremely dangerous. Greener, safer explosives are needed, the researchers say.
To meet this need, Klapötke and Sabate turned to a recently explored class of materials called tetrazoles, which derive most of their explosive
energy from nitrogen instead of carbon. They identified two promising tetrazoles: HBT and G2ZT. The researchers
developed tiny “bombs” out of these materials and detonated them in the laboratory. The materials showed less sensitivity to shock than
conventional explosives and produced fewer toxic products when burned, the researchers say.
--------------------------------------------------------------------------------
Journal reference:
Klapötke, Thomas M. and Sabaté, Carles Miró. Bistetrazoles: Nitrogen-Rich, High-Performing, Insensitive Energetic Compounds. Chem. Mater., 2008
doi: 10.1021/cm703657k
|
Here is HBT:
http://en.wikipedia.org/wiki/HBT_(explosive)
and here is G2ZT:
http://en.wikipedia.org/wiki/G2ZT
There is no structure in the Wiki article.
[Edited on 6-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
"Bis(3,4,5-triamino-1,2,4-triazolium)-5,5'-azotetrazolate."
Looks dodgy, unless "1,3,5-triamino-1,2,4-triazolium" was what was intended.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
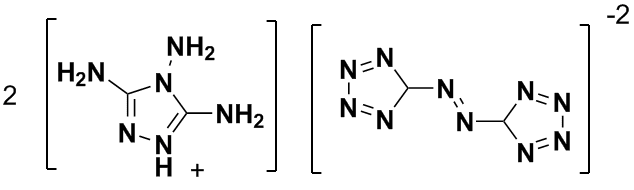
| Quote: | Originally posted by sparkgap
"Bis(3,4,5-triamino-1,2,4-triazolium)-5,5'-azotetrazolate."
Looks dodgy, unless "1,3,5-triamino-1,2,4-triazolium" was what was intended.
sparky (~_~) |
I think you are correct that he has prepared a salt. Here is a snippet from the Web on the makeup of G2ZT:
| Quote: | | 3,4,5-triamino-1,2,4-triazolium cation similar ..... “Salts of 5,5’-Azotetrazolate” Eur. J. Inorg. Chem. 2002, 834 |
There is discussion of these salts in this thread: http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a....
I just sent an email request to Herr Prof. Dr. Klapoetke for a pdf or a graphics file to get the correct structure & prep. If I receive anything
back I'll post it here. In the meantime here is my revised version the composition of G2ZT:
And here is a Google Book reference of the 5,5'-azotetrazolate dianion: http://tinyurl.com/5uf4np
[Edited on 6-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Can't get this as a pdf for some reason, it just will not load. Odd. Here is some c/p from the HTML article.
Preps:
Synthesis of 5,5′-Hydrazinebistetrazole (HBT). Sodium 5,5′-azotetrazolate pentahydrate (50.00 g, 166.63 mmol) was dissolved in 1.4 L of
water before an excess of magnesium powder (28.71 g, 1.18 mol) was added to the yellow solution. The reaction mixture was refluxed for 6 h and left to
cool to room temperature under a stream of nitrogen. The excess magnesium powder was filtered along with some magnesium hydroxide, which precipitated
on cooling (this operation has to be carried out quickly in order to prevent oxidation of sodium 5,5′-hydrazinobistetrazolate) into a sidearm
flask containing 160 (1.58 mol) mL of 6 M HCl (once the filtrate came into contact with the HCl no oxidation of the hydrazino-bridged compound was
observed). A white solid immediately formed and the sidearm flask was shaken thoroughly to favor the precipitation of the product. The slightly yellow
powder was filtered off and washed thoroughly with water and with acetone, yielding the desired product as a white powder (26.64 g, 95.2%). Crystals
of the compound suitable for structure determination were grown by dissolving a small amount of the solid in boiling water and letting to cool slowly.
C2H4N10 (calcd/found, %): C, 14.29/14.27; H, 2.40/2.58; N, 83.31/82.68. Mp (Büchi B-540, uncorrected): 206.9–208.2 °C (decomposition). 1H NMR
(DMSO-d6, 400.18 MHz, 25 °C, TMS): δ 9.66 (4H, s, NH). 13C{1H} NMR (DMSO-d6, 100.63 MHz, 25 °C, TMS): δ 159.68 (s, CN3); Decomposition
Experiments. MS (EI): m/z = 12 (0.1, C+), 14 (0.6, N+), 16 (1.2, NH2+), 17 (3.6, NH3.+), 26 (3.2, CN+), 27 (20.4, HCN.+) and 28 (100.0, N2.+). IR
(Gas): Δν (cm−1) 3332 (w, NH3), 3336 (s, HCN), 3282 (s, HCN), 2168 (w, HCN), 2102 (w, HCN), 1621 (m, NH3), 1431 (w, HCN), 1380 (w,
HCN), 965 (vs, NH3), 926 (vs, NH3), 732 (s, HCN), 710 (vs, HCN), 679 (s, HCN).
Synthesis of Bis(3,4,5-triamino-1,2,4-triazolium) 5,5′-Azotetrazolate (G2ZT). To an aqueous solution of sodium azotetrazolate pentahydrate
(31.01 g, 103.25 mmol) in 150 mL of hot water was added 3,4,5-triamino-1,2,4-triazolium bromide (40.185 g, 206.50 mmol) in 100 mL of hot water. The
mixture was stirred and boiled for 3 h and the title compound, only slightly soluble in hot water, started to precipitate. After slow cooling to room
temperature, the yellow solid was filtered, washed with a small amount of cold water and methanol, and air-dried (37.24 g, 91.6%). Crystals suitable
for X-ray analysis were obtained by recrystallization from water of a small amount of the compound. C6H14N22 (calcd/found, %): C, 18.28/18.22; H,
3.58/3.68; N, 78.15/77.95. Mp (Büchi B-540, uncorrected): 211.3–212.4 °C (decomposition), 1H NMR (DMSO-d6, 400.18 MHz, 25 °C, TMS): δ 6.60
(4H, s, C-NH2), 5.58 (2H, s, N-NH2). 13C{1H} NMR (DMSO-d6, 100.63 MHz, 25 °C, TMS): δ 173.24 (2C, [C2N10]2− , 150.31 (2C, C-NH2); Decomposition Experiments. MS (EI): m/z = 12 (0.6, C4+), 13 (0.3,
CH3+), 14 (3.4, CH22+, N+), 15 (0.4, CH3+), 16 (10.0, CH4.+, NH2+), 17 (13.6, NH3.+), 26 (5.8, CN+), 27 (38.0, HCN.+), 28 (100.0, N2.+), 31 (0.1,
NH2NH+), 32 (0.2, NH2NH2.+), 38 (0.0, CCN3+), 39 (0.0, CHCN2+), 40 (0.1, CH2CN+), 41 (0.2, CH3CN.+). IR (Gas): Δν (cm−1) 3450 (w,
NH3), 3333 (s, HCN), 3285 (m, HCN), 2166 (w, HCN), 2102 (w, HCN), 1623 (s, NH3), 1431 (w, HCN), 1382 (w, HCN), 964 (vs, NH3), 925 (vs, NH3), 731 (m,
HCN), 714 (vs, HCN), 681 (m, HCN), 667 (w, NH3), 624 (vw, NH3). , 150.31 (2C, C-NH2); Decomposition Experiments. MS (EI): m/z = 12 (0.6, C4+), 13 (0.3,
CH3+), 14 (3.4, CH22+, N+), 15 (0.4, CH3+), 16 (10.0, CH4.+, NH2+), 17 (13.6, NH3.+), 26 (5.8, CN+), 27 (38.0, HCN.+), 28 (100.0, N2.+), 31 (0.1,
NH2NH+), 32 (0.2, NH2NH2.+), 38 (0.0, CCN3+), 39 (0.0, CHCN2+), 40 (0.1, CH2CN+), 41 (0.2, CH3CN.+). IR (Gas): Δν (cm−1) 3450 (w,
NH3), 3333 (s, HCN), 3285 (m, HCN), 2166 (w, HCN), 2102 (w, HCN), 1623 (s, NH3), 1431 (w, HCN), 1382 (w, HCN), 964 (vs, NH3), 925 (vs, NH3), 731 (m,
HCN), 714 (vs, HCN), 681 (m, HCN), 667 (w, NH3), 624 (vw, NH3).
Product:
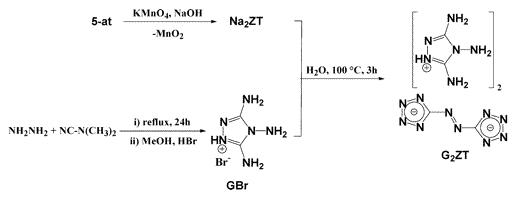
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by The_Davster
Can't get this as a pdf for some reason, it just will not load. |
I just received this from the author.
Attachment: 403.pdf (956kB)
This file has been downloaded 4142 times
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
A recent article on the use of some of these high nitrogen compounds in pyrotechnics.
From the usual group which seems behind all these nifty compounds
Attachment: green pyro.pdf (1.2MB)
This file has been downloaded 3911 times
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
New energetic from Prof Dr Klapötke
| Quote: | Phys Chem Chem Phys. 2008 Aug 7;10(29):4340-6. Epub 2008 Jun 9.
Triaminoguanidinium dinitramide-calculations, synthesis and characterization of a promising energetic compound.
Klapötke TM, Stierstorfer J.
Energetic Materials Research, Department of Chemistry and Biochemistry, University of Munich (LMU), Butenandtstr. 5-13, D-81377, Germany.
tmk@cup.uni-muenchen.de.
The highly energetic compound 1,3,5-triaminoguanidinium dinitramide was prepared in high yield (82%) according to a new synthesis by
the reaction of potassium dinitramide and triaminoguanidinium perchlorate. The heat of formation was calculated in an extensive computational study
(CBS-4M). With this the detonation parameters of compound were computed using the EXPLO5 software: D = 8796 m s(-1), p = 299 kbar. In addition, a full
characterization of the chemical properties (single X-ray diffraction, IR and Raman spectroscopy, multinuclear NMR spectroscopy, mass spectrometry and
elemental analysis) as well as of the energetic characteristics (differential scanning calorimetry, thermal safety calorimetry, impact, friction and
electrostatic tests) is given in this work. Due to the high impact (2 J) and friction sensitivity (24 N) several attempts to reduce these
sensitivities were performed by the addition of wax. The performance of was tested applying a "Koenen" steel sleeve test resulting in a critical
diameter of >/=10 mm.
|
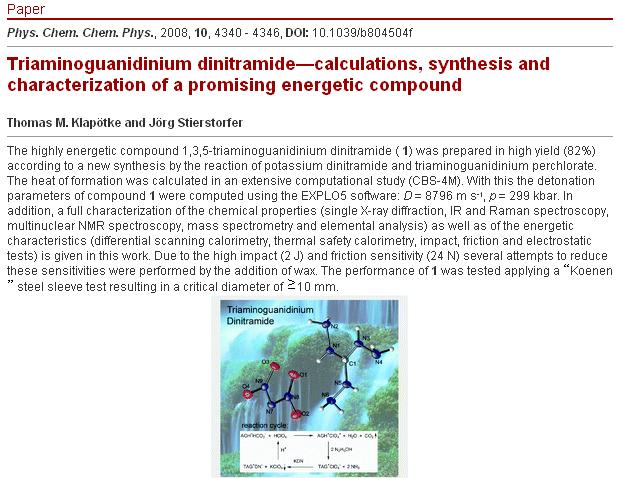
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
BPTAP
Thermally-stable inner salt compounds have been known for some time. Los Alamos recently had a U.S. Patent Application published that describes the
preparation of the new secondary explosive BPTAP: http://v3.espacenet.com/origdoc?DB=EPODOC&IDX=US20081850...
Additional info on BPTAP & other recent developments in explosives is found here: http://www.pnas.org/content/103/27/10322.full
The process flow & final structure are shown here:
[Edited on 3-9-2008 by Ritter]
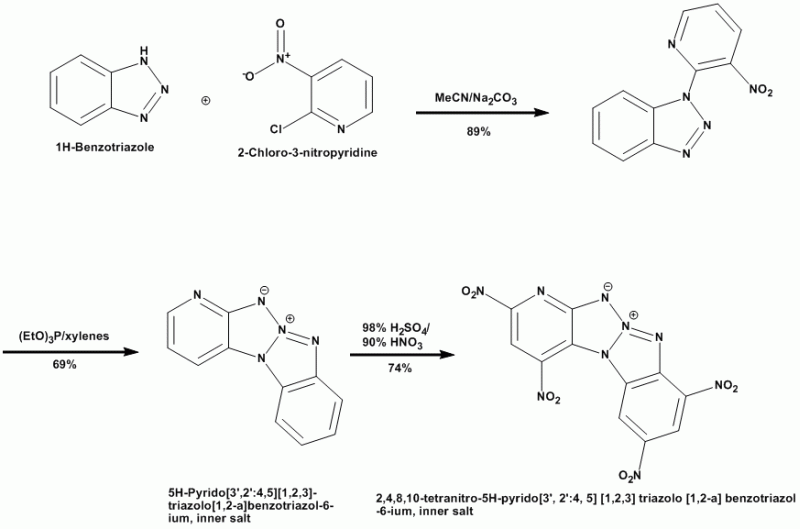
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Does anyone have synth., or other info on the new energetic developed in LANL by D Chavez?
Six carbons, two nitro and four nitrate groups would make for good O.B and its density, too, is quite high.
That and the low m.p. makes it sound particularly interesting.
[Edited on 25-12-08 by The_Davster]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
It looks like a very interesting little compound.
Here it is:
[Edited on 25-12-08 by The_Davster]
Attachment: Synthesis of an Energetic Nitrate Ester.pdf (376kB)
This file has been downloaded 4544 times
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Many thanks, 497; it's great to have a detailed synthesis at hand.
The OB at zero makes it as powerful as nitroglycol and predicted brisance is higher(high density), so we'll hear a lot more of this explosive.
ETN looks pedestrian, by comparison.
What still commends ETN, though, is its simplicity.
After experience of HMTD, cyclonite, NGL and methyl nitrate, ETN's next on the list.
[Edited on 25-12-08 by The_Davster]
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
"BTW, the unfair "redneck" reference to Mega's site was a stupid response to the anti-Obama vitriol I saw there.
I hope it didn't offend."
To your credit you did say it was unfair, but then you try to rationalize it. If it was an ant-Bush diatribe, would you feel the need to interpret the
politics of the site for us?
|
|
|
Axt
National Hazard
   
Posts: 856
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by hissingnoise
Does anyone have synth., or other info on the new energetic developed in LANL by D Chavez?
Six carbons, two nitro and four nitrate groups would make for good O.B and its density, too, is quite high.
That and the low m.p. makes it sound particularly interesting.
RS. (redneck's science?) has a thread on it, but I can't access synth. details. |
Very simular structure to that found in US patent 2381406, though without the methylene bridge. Its description (Six carbons, two nitro and four
nitrate groups) also fits the nitration product of dinitroinositol I mentioned on the second page of this thread which supposedly also posesses
performance simular to HMX.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Axt, your reference to the second page got me thinking I should read the entire longrunning thread, once-and-for-all.
I've gotten through (phew!) a large section, and now I'm poly-nitrogened out. . .
'Think I'll go lie down for a while---say, a week, maybe.
'Seriously, there seems to have been a veritable explosion of new energetics in the last several years.
How many, though, will make it into actual service?
|
|
|
| Pages:
1
2
3
4
5
..
23 |
|