| Pages:
1
2
3
4
5
..
15 |
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
http://pyrobin.com/files/LPH.pdf
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | For sure was tested mixture Li8 + Cu8 50:50. Brisance is bigger than CHP, but weaker than at others on picture. Pretty surprisingly is, that Li8 is
difficult fired as free heap. During a 2 second goes out after ignition. But detonation pressure of Li8 is the highest of all examined mixtures. I
don't know of any other primary mixture (or primary-secondary) that will go out on its own if you ignite it. For reliable DDT of course Li8 require
solid metal cavity. From unknown reason is surface of crater from Li8 pretty smooth. Against all others. And after test was this crater as only one
clear, without black residuum. Explosive fumes were also odorless only at Li8. But that has been mentioned before.
List in brizance order:
ETN ...........5,34 mm
Li8..............4,17 mm
Cu8............4,10 mm
Li8/Cu8......3,67 mm
CHP...........3,46 mm
|
Yes there are primary/secondary mixtures that can DDT and self extinguish… they are the holy grail because of safety, a stray spark unconfined does
nothing. Congress LL, this is a big invention  )). I am genuinely jealous, I wish
it were me instead, that’s how big this is )). I am genuinely jealous, I wish
it were me instead, that’s how big this is 
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Lithex
No need to be jealous. Drying of the Li8 mixture at 150 C could end in an explosion. Fortunately, this did not happen. Drying and scraping and
crushing at 150 C were repeated at least 30 times. No accident. Fortunately. But not everyone wants to be an astronaut. Because it's a very risky way
up there. A professional chemist would never heat a perchlorate ligand with hexamine to 150 C. On a metal surface. Plus no thermometer. The
temperature is only estimated. But it must be such that the crystalline barrier is overcome and the ligand gives up all the water. This is certainly
possible at lower temperatures in a vacuum. Maybe only at 50 C. This ligand has been known for many years. But no one dared to deprive him of water.
Either way, vacuum or classic. This happened for the first time 1.1. 2022. At the temperature at which you fry the eggs. Until it turns black.
A lower temperature is used for Cu8, estimated at 80 - 100 Celsius. But pungent gas is produced, which is uncomfortable. There is basically nothing to
feel about Li8. You just have to pray. And occasionally set the pan and cool a little. Especially when crushing larger agglomerates 2x2 mm. For
example, at 100 Celsius. And then increase again to 150 C for drying. And repeat the whole procedure until a fine powder is formed. Which can easily
get rid of the last remnants of water. The grain must be almost like powder, ie less than 0.1 mm. A well-dried pile of 100 mg burns whole, without
residue. Slowly, but it burns. In aluminum foil V-shaped closure, it burns quite quickly, about 10 - 15 cm / s. Another thing: Few people have LiClO4
to do such an experiment at all. It is pretty expensive chemical. 100g over 100 Dollars. This dramatically reduces the likelihood that someone will
try or have tried it before. Like almost all inventions, it is usually a more coincindences together. No need to envy, be jealous.
It was a coincidence. And nothing bad happened....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
What about lithium nitrate. The king, same density far more oxygen. I owe this knowledge to a young Enthusiastic friend.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Lithium nitrate maybe will work. You can try it. Or someone. Also sodium perchlorate provide interesting properties. In table is writte zero. But not
is exact truth. NaP + hexamine ligand also can detonate as DDT substance in solid cavity. But his power is weak. Also sometimes worked, sometimes not
worked. Shortly is unreliable. Yet. NaP is easily available
and maybe will work with ETN in one solid cavity. In some ratio. Also with adding alu powder. Or small % diethyleneglycol.
Because NaP + DEG is powerful EM. But not primary, is it insensitive secondary. In parts: 75 NaP + 25 DEG + 5 - 15 H2O. Maybe without water, but with
hexamine + DEG (small amount about 1 -5 % I estimate) It will works. It was not tested....
NaP I see a like interest way for research. Is cheap, can create ligand with hexamine. Maybe from it some will.
--------------I specify the temperature for drying Li8 to anhydrite. The minimum is 150 C, the maximum 165 C.---------------
Another interesting fact is that there is no need to add water when mixing LiP + Hexamin. If LiP dihydrate is used, simply strew both components onto
the pan. Internal water from LiP will take care of dissolution. The dihydrate therefore serves as a fairly accurate thermometer for measuring surface
temperature. And it shows the minimum temperature required for mixing.The performance of such a mixture after drying is the same (same holes) and the
preparation is faster. As soon as the dihydrate crystals start to melt, the heating is stopped. And at this temperature, mixing and drying are
continued.
During 10 - 15 minute is done. Arise very fine white powder. Li8 is more white than method with adding water. Used ratio 2,98 LiP x 2 H2O + 0,69 hex
x 0 H2O.
[Edited on 25-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
There are others in which one or both is a minor component, <10%. I'm unaware of other patents using (per)chlorate and hexamine as main oxidizer
and fuel, other than airbag composition e.g. 15.7% hexamine, 47.7% AP, 36.2% Na nitrate, balance fumed silica.
Quote: Originally posted by Laboratory of Liptakov  | | Another thing: Few people have LiClO4 to do such an experiment at all. It is pretty expensive chemical. 100g over 100 Dollars. This dramatically
reduces the likelihood that someone will try or have tried it before. Like almost all inventions, it is usually a more coincindences together.
|
"Lab Depot" charges $63.60 (not including $19 shipping) for 25 g LiCl...it's easily made even without the acid, from LiCl and the Na salt (even
starting from HTH). It's mentioned in that airbag patent.
It also forms a tetrahexamine hexahydrate, which had to be brought up simply because one of the authors for this 1932 Bull. soc. ref. is named
Krawczynski.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Lesson: Lab Depot still more dramatically reduces the likelihood that someone will try make LiClO4....
Maybe will better HClO4 from NH4ClO4/HNO3/HCl. In method Horace C. Adams. And then HClO4 + Li (or LiOH)
[Edited on 25-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
An acid-base reaction between LiOH + HClO4 was successfully performed. The stoichiometric ratio is 6.8 g HClO4 + 1.62 LiOH. Practice: 11g HClO4 68% +
20g dH2O as solution A. 2.92 LiOH monohydrate + 30g dH2O as solution B. Solution B is added dropwise to solution A. The reaction proceeds without
splashing and almost without heating. After the addition of 25 g of solution B, the pH must be measured frequently. Solution equilibrated dropwise to
pH 7 neutral. After evaporation of water, the yield was 8.39 g of LiClO4 x 0 H20. Finální teplota povrchu 165 C. Approximately 0,5% was mechanical
losses. From this Li8 (Lithex) was synthesized.
Its energy properties are the same as when using factory LiClO4.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by S.C. Wack  |
"Lab Depot" charges $63.60 (not including $19 shipping) for 25 g LiCl...it's easily made even without the acid, from LiCl and the Na salt (even
starting from HTH). It's mentioned in that airbag patent.
|
Meanwhile in other shops...
5.48 Euros for 25 grams of the perchlorate from Chemcraft.
500 grams for 73 Eu.
They also have Li metal and HCLO4 for those who like to make everything from scratch.
10 grams for 5 Euros from Onyxmet.
Best for last: LiCl x H2O for 70 Euros a kilo (compared with the 63$ for 25 grams from Lab Direct!)
These businesses are a huuuge opportunity for the researcher with little needs and little means.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Lithex preparation
Good links, thanks Herr Haber....
LiCl https://onyxmet.com/index.php?route=product/product&path...
HClO4 https://chemcraft.su/product/25786
LiClO4 https://chemcraft.su/product/23226 19 USD / 100g very cheap
NaClO4 https://chemcraft.su/product/25565
hexamine https://chemcraft.su/product/25713
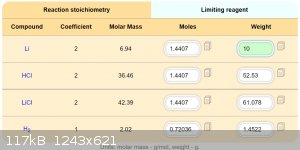
Maybe this should by works.
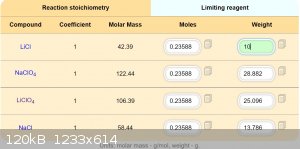
Solubility compounds in 100g acetone:
LiCl ................1,2g
NaClO4.........52g
LiClO4.........137g
NaCl............ 0,000042g
I estimate, that double recrystallization provide pretty pure LiClO4.
Better of course will buy directly LiClO4 by 19 USD
[Edited on 27-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
LiClO4 is proving to be a promising oxidant. For example, LiClO4 anhydride 70% + diethylene glycol (DEG) 30% provides an interesting energy substance.
This substance has a melting point of 70 Celsius.
In the liquid state, it is sensitive to impact, similar to nitroglycerin. And it gives similarly loud blows. But once it solidifies, it is impossible
to detonate the mixture. Even with a strong hammer blow, there is no detonation. The mixture can be heated to 165 Celsius for a long time without
changing the properties. It can be allowed to solidify and reheat. It will most likely be hygroscopic which is difficult to detect. On V-shaped
aluminum foil burns with an effective, regular red-violet flame. No jets and irregularities in burning. But speed of burning is relatively high.
This could be an interesting alternative for small rocket engines. In the liquid state, the viscosity is like a thin oil. This would allow casting at
low temperature. Due to the lightness of Lithium, such a mixture could have a high specific impulse. The disadvantage will be the price of such a
composition.
A mixture of LiClO4 + DEG as base + Alu powder + hexamine or another similar composition based on LiP + DEG is also possible. Not examined.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
That was the point. It was a commentary on chemicals offered for high prices, specifically LL's buy, not a reflection of the actual market here.
It's true that the chloride and esp. the carbonate costs 3-5x more than they did 20 years ago and will soon double again. I would not expect chlorate
and perchlorate to be shipped inexpensively. The acid is unchallenging to make, as is LiP without the acid, Cl, or Pt, with or without thermal or
chemical processes, from various perchlorate, chlorate, and hypochlorite sources, starting at any point, using solubility characteristics to
advantage. (the ammonium-alkali-alkaline earth oxychlorides being all connected to each other in this way)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Every hobby costs something. For example, aircraft modeling, drones, RC planes and the like cost about 5x to 10x as much as unit-level research of up
to tens of grams in the field of chemistry. So that one can have fun entires days. My life experience shows that chemistry is one of the cheapest
hobbies ever...
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
I still wonder what the irritating vapors are when making the copper perchlorate version. I also wonder about the molecular structure of the finished
product. It might help us to understand long term stability or help us discover similar combinations involving nitrate or chlorate salts.
The diethylene glycol/lithium perchlorate melt is another example of a concept I have been very interested in. In fact, it might be the best
demonstration of it ever.
A solution of a fuel and oxidizer completely dissolved in each other is much more likely to detonate than a mixture of two powders or even a liquid in
a powder. The molecules in a solution are literally side by side and can react almost as fast as a molecular HE.
Acetone dissolving more than its own weight of lithium perchlorate seems like another good candidate. I haven't done math for how close that is to
oxygen balance/how much energy it could produce, but a saturated acetone/perchlorate solution might be cap sensitive.
Another promising mixture like this is calcium nitrate and methyl alcohol. In this case you could only get maybe half the required oxidizer to
dissolve so you would need to gel the mixture and then suspend very fine calcium nitrate particles into it. The double salt/hydrate
5[Ca(NO3)2].NH4NO3.H2O is sold over the counter here as a tomato fertilizer. Methanol is sold as an automotive fuel additive. It's about 7 euros for a
2 kg bag of the fertilizer and about 2 euros for 500ml of methanol.
I haven't actually tested this one because I haven't found a good way of gelling methanol, and I also don't have the other reagents or equipment to
make or test an initiating charge at this point.
I'd love to find a chlorate that makes a somewhat stable primary or DDT mixture, and also one that is soluble in meaningful quantities in a liquid
fuel.
Put that in your pipe and smoke it!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Lithex
The molecular structure of Cu8 is still a mystery. Because it is prepared from slightly acidic Cu (ClO4) 2. After the reaction, the acidity decreases
because an acid-base reaction occurs between hexamine and HClO4. Similar to preparing hexamine diperchlorate.
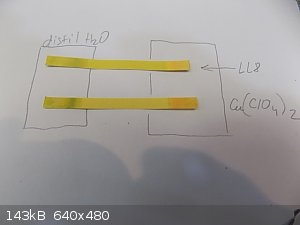
Acidity in Cu8 (LL8) is not some horrible. But is it product of alchemy yet.
Li8, ie Lithex, looks more promising. Pure neutral LiClO4 is used and the Lithex structure will almost certainly be more stable. Just because it can
withstand an hour of heating at 150 C.
There are certainly dozens of mixtures with calcium, nitrate and who knows what else. Maybe some from them will works.
But this fiber deals with functional and tested mixtures, especially based on LiClO4.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Once I bought 12 kg of hexamine. At that time it was extremely cheap. But me, an idiot, placed it into the storage with a leaky roof. Next year I
found only an empty box.
Women are more perilous sometimes, than any hi explosive.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Herr Haber  | Quote: Originally posted by S.C. Wack  |
"Lab Depot" charges $63.60 (not including $19 shipping) for 25 g LiCl...it's easily made even without the acid, from LiCl and the Na salt (even
starting from HTH). It's mentioned in that airbag patent.
|
Meanwhile in other shops...
5.48 Euros for 25 grams of the perchlorate from Chemcraft.
500 grams for 73 Eu.
They also have Li metal and HCLO4 for those who like to make everything from scratch.
10 grams for 5 Euros from Onyxmet.
Best for last: LiCl x H2O for 70 Euros a kilo (compared with the 63$ for 25 grams from Lab Direct!)
These businesses are a huuuge opportunity for the researcher with little needs and little means. |
Does chemcraft ship world wide.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
This is comedy gold right here  )) ))
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | LiClO4 is proving to be a promising oxidant. For example, LiClO4 anhydride 70% + diethylene glycol (DEG) 30% provides an interesting energy substance.
This substance has a melting point of 70 Celsius.
In the liquid state, it is sensitive to impact, similar to nitroglycerin. And it gives similarly loud blows. But once it solidifies, it is impossible
to detonate the mixture. Even with a strong hammer blow, there is no detonation. The mixture can be heated to 165 Celsius for a long time without
changing the properties. It can be allowed to solidify and reheat. It will most likely be hygroscopic which is difficult to detect. On V-shaped
aluminum foil burns with an effective, regular red-violet flame. No jets and irregularities in burning. But speed of burning is relatively high.
This could be an interesting alternative for small rocket engines. In the liquid state, the viscosity is like a thin oil. This would allow casting at
low temperature. Due to the lightness of Lithium, such a mixture could have a high specific impulse. The disadvantage will be the price of such a
composition.
A mixture of LiClO4 + DEG as base + Alu powder + hexamine or another similar composition based on LiP + DEG is also possible. Not examined.
|
Yes yes! Remember our test from a few years ago?! The casting idea is excellent!! Of course zirconium could be added… but if sensitivity is that of
NG mineman scared
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
LiDEG
Of course zirconium could be added… but if sensitivity is that of NG mineman scared.... ... ...
Sensitivity is high only over 70 C as liquid.
But if it worked (with unknown ingredients under 70C), casting into the cavity would be possible without pressing. LiDEG will be further investigated.
Further trials have shown that Lithex is pretty resistant. Stress test: Lithex was dissolved in a concentrated solution of NH4OH 25% aq for 24 hours
1g / 10g. The solution was then poured onto a pan and dried at 165 ° C. The temperature was maintained at 120 ° C for 2 hours. Its properties did
not change. The resulting hole in the 2 mm sheet is still 7.8 - 8 mm. Under standard conditions of the whole set, of course.
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Pretty much, check their web site. There are a few places they won't ship to. I have purchased from them many times with delivery to Australia.
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by B(a)P  |
Pretty much, check their web site. There are a few places they won't ship to. I have purchased from them many times with delivery to Australia.
|
They seem way better than the polish oxnymet. Are there any better sites even? Nothing like this but a Chinese version?
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MineMan  |
They seem way better than the polish oxnymet. Are there any better sites even? Nothing like this but a Chinese version? |
Not that I know of.
Both are great sources for small amounts of very pure chemicals and a gold mine for element collectors.
I have found that both ship very professionally and never felt the need to repackage anything they had sent.
Cant say the same with China !
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Preparation LITHEX here: https://www.youtube.com/watch?v=BubkrGVEwF8&t=2s
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Yahhh  any update on increasing the density to the holy 2.0g/cc? any update on increasing the density to the holy 2.0g/cc?
|
|
|
| Pages:
1
2
3
4
5
..
15 |