| Pages:
1
2
3 |
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Boffis thx for reviving this thread. I have hundreds of wild cherry trees in my forest (yet small, I planted them there only 10 years ago). I grow
sweet corn every year in my garden and there are plenty of huge agricultural fields with common corn around (to feed cattle with fermented plants =
silage during winter) - no problem with corncobs.
I've bought d-ribose 100 g for 119 CZK which is 4,5 EUR... I guess it was sale-out of pharma grade compound near to its expiration date as it is not
available there anymore and strange low price:
https://fichema.cz/241-d-riboza
When I find something very cheap I just buy it for possible future use even without actual ideas what to do with it. Now I know 
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Fery, I hope to get my prepub posting up today. But yes the price of D-ribose in the UK now is as low as £25 per Kg (about 29 euros), xylose was
even cheaper but the company that sold it has disappeared. Prunus sps. gums look like an ideal source for small scale production though if you have a
ready source. If you do try it let us know how you get on using it as a source directly.
Another possible source that has just occurred to me is xylitol. I don't think this pentol will give furfural directly but I was wondering if it can
be oxidised to a pentose with say hypochlorite or H2O2/Fe. There would be no need to isolate the sugar. I may try this as xylitol is cheap in our
local supermarket.
[Edited on 1-1-2021 by Boffis]
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Boffis, good and cheap source!
xylitol costs here cca 10 EUR per 1 kg pharma/food grade
https://fichema.cz/265-xylitol-brezovy-cukr
I found an information about methyl furoate:
https://sci-hub.st/10.1002/14356007.t11_t02
methyl 2-furoate [611-13-2]: fruity, mushroom-like odor
and the furfural = 2-furaldehyde [98-01-1]: freshly baked bread odor
wow! I love bread, it is substantial portion of my food! Looking forward the synthesis...
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Fichem.cz only ships and sells within CZ and SK. Sad, because they have some really good chemicals and prices.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
OK I've posted my full write-up on the preparation of furfural from various source over in the "Prepublication" section.
Yesterday I also ran a larger scale preparation from xylose using 150g with 440g of salt in 1.5 litres of solution and over 7 hours recovered 51.5g of
crude furfural which is about 53.5% so almost exactly the same recovery as before in spite of the increased ratio of salt to xylose and the larger
scale.
Anyone any ideas on the oxidation of xylitol to a pentose?
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have now repeated the larger scale preparation using 150g of D-ribose in stead of xylose. I used exactly the same procedure as above but the yield
was only 25.2g and no further furfural passed over after 3 hours. It seems that ribose is not a good source for large scale furfural preparations by
this route.
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Boffis, you used 25 g ribose in your first synthesis which yielded 10,2g of fural and now 150g of ribose which is 6x more and yield 25,2g. Did the
distillation last the same time in both preparations? If this second synthesis lasted approx 2x longer (because of bigger flask) then perhaps it is a
hint that fural has to be distilled out of the reaction mixture as quickly as possible... just guessing... if this is true then even returning water
saturated with fural back into the distillation flask is contraproductive, perhaps collecting distilled water with fural and then put this into dean
stark trap apparatus with a hope that without acid the fural won't decompose on the second distillation? During the reaction instead of returning
water saturated with fural from the condensate back into the distillation flask just dripping there clear water from dropping funnel as a lazy
solution or steam as an advanced solution?
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Fery, Yes. In both cases with xylose I had to run the reaction for about 7 hours to get to a point where no more furfural droplets formed. I have
discovered that it is important to keep the reflux rate as low as possible. I think that this is because furfural is slightly soluble in warm water
and the higher the reflux rates it is simply carried back into the boiling flask. I have thought about using external steam injection (ie true steam
distillation) to increase the rate of extraction but this then gets back to the old problem of recovering the furfural from a large volume of aqueous
distillate. The replenishment of the water with fresh water and collecting all of the distillate clearly has the same problem.
With ribose at the 25g scale distillation lasted 5 hours but at the 150g scale distillation was over in only 3 hours and the yield was lower (26 as
against 60+%). The problem may be that as U235 commented, ribose reacts more quickly and can't be removed fast enough so ends up being decomposed.
Reducing the reflux rate makes this worse but conversely increasing the reflux rate simply returns more to the reaction flask and hence decomposition.
I am currently setting up an experiment to carry out tomorrow using 100g of very strong ribose solution and add it slowly to boiling salt and acid.
I'll let you know how I get on  . .
One final thought. Does anyone know of a use for the black humic material? 
[Edited on 4-1-2021 by Boffis]
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
While emptying out the flask from one of the reaction I found these beautiful hopper salt crystals in the black residue. They are about 9mm on edge,
there were larger ones but they were not so cute.
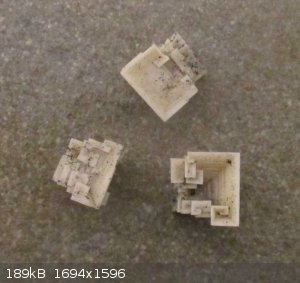
[Edited on 11-1-2021 by Boffis]
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have now run two attempts at Fenton oxidation of xylitol (Fe2+ catalyzed H2O2 oxidation) and then distilled the product without isolation of the
xylose. The first experiment used a 1:1 Molar ratio of xylitol to H2O2 and 50g of xylitol gave just 3.37g, I was expecting 15-18g. The next run I used
a ration of 1:2 but the yield fell to zero.
I then tried Fe3+ catalyzed H2O2 oxidation of sodium gluconate to arabinose followed by acidification and distillation; again the yield was
practically zero. (aniline test was strongly positive on the aqueous distillate but no oil separated).
Then I tried oxidising glucosamine HCl with hypochlorite solution to give arabinose and then distill this with salt and HCl. Again the yield was zero
though the aqueous phase gave a positive test for furfural.
I am currently following a published procedure to oxidize 187g of sodium gluconate to the letter and then intend to isolate the arabinose as a solid.
I will try then to distill this material.
At present it still looks like d-ribose, available in the UK for 3 to 4 pounds sterling per 100g which gives about 30-35g of furfural if distilled in
small batches is the best amateur route. Xylose is a better source on a larger scale because its cheaper but it is less readily available OTC.
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Boffis, I've bought something else but from this family - furfuryl acetate, the ester of furfuryl alcohol and acetic acid.
From here:
https://shop.es-drei.de/ester/694/essigsaeurefurfurylester-m...
here info about its scent:
http://www.thegoodscentscompany.com/data/rw1017931.html
sweet fruity banana horseradish
For me it scents like horseradish, but the scent is not unpleasant, I would rather say neutral. I do not recognize sweet / fruity / banana in it. I
was unable to identify the scent for the first time, I only remembered that I already scented it sometimes. Only after reading the scent description I
suddenly remembered. My father grated horseradish from his garden a lot of times, it was especially served with ham and meat around Easter. I'm
growing horseradish in my garden too since previous year, but I did not yet dug its roots out of the soil, maybe I'll try in few weeks. Strange that I
remembered the scent but was unable to assign the source from which I knew it. And only after reading info I remembered. We have memory for images,
sounds, touches (body map + type of sensation like touch/cold/warm/pain/vibration etc), tastes and scents.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Mmmm I'm trying to imagine the smell of horseradish. I don't find it has a smell as much as a mustard like sensation in the nose! 
I am preparing bromine at present to prepare some mucobromic acid from my furfural. I now have about 300ml so I should have plenty left (I'm going to
use the Org. Synth. route half scale.). One of the things I might try is the oxidation to furoic acid. I have already prepared furoin and furil and
posted the preparation of them somewhere on SM; so ethyl furoate shoul;d be a possibility.
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Boffis, grated horseradish has quite unpleasant effect in copious secretion from nose and flood of tears. Furylacetate has only the scent without
the lachrymator allyl isothiocyanate present in horseradish. When you buy ready to eat grated horseradish in a jar, it is too weak, but when you try
to grate fresh root, its power is demonstrated meters faraway.
When you have 2-furaldehyde, Cannizzaro reaction produces alcohol + acid, you can separate 2-furfurylalcohol by extraction into organic solvent while
2-furoic acid stays in water phase in a form of Na salt. But that you know much better than me, you already posted nice Cannizzaro reaction
experiment.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have thought about Cannizzaro's reaction but its not very efficient with furfural because furfurol tends to resinify. Since I am more interested in
the acid I may try oxidation of furfural.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Good prices on sugars. Nice clean starting material. Me? I'm in the land of "Corn on the Cob". At the right 4th of July picnic, I could salvage
maybe 50 gallons of Corn Cobs. According to Org. Syn., that's a lot of Furfural.
Cannizaro is a good start. 50% yield of the acid, right from the get go. Then, you can oxidize the Alcohol to the Acid. Though, as the Wicked
Witch Said: "These things must be done delicately" "So as to not spoil the magic!"
Conjugated system. And, the problem with oxidizing agents, is reining them in. Need to be wary of those double bonds.
I'm gonna go back and study this whole thread.
[Edited on 17-3-2021 by zed]
[Edited on 17-3-2021 by zed]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Marginally related: here’s an old paper on the preparation of furfuryl furoate.
Attachment: ja01682a035.pdf (150kB)
This file has been downloaded 294 times
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, I checked back a decade or so, and this is an old thread.
It no longer seems to contain an active link to this Corn Cob procedure.
Said procedure, containing pointers on how gently you've got to handle Furfural.
It seems to "remember" abuse, and avenge itself later.
http://www.orgsyn.org/demo.aspx?prep=CV1P0280
http://www.orgsyn.org/demo.aspx?prep=CV1P0276
[Edited on 18-3-2021 by zed]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by Boffis  |
At present it still looks like d-ribose, available in the UK for 3 to 4 pounds sterling per 100g which gives about 30-35g of furfural if distilled in
small batches is the best amateur route. Xylose is a better source on a larger scale because its cheaper but it is less readily available OTC.
|
I believed this too, until about five minutes ago. It is certainly true that xylose is not sold at the grocer. But it turns out that polymers of
xylose are not nearly as rare. The popular supplement psyllium (Metamucil) consists primarily of arabinoxylans (60% w/w), polymers of
primarily xylose with some arabinose, these both being pentoses. And here we are as OTC as it gets! Available in the UK from at least one retailer for
£21/kg and cheaper in the US.
EDIT: Furfural is effectively oxidized by oxygen in the presence of a copper manganate (CuMn2O5) and NaOH:
https://www.sciencedirect.com/science/article/pii/S246802572...
Rxn conditions were 120 C, 1 Mpa O2 with a low catalyst loading (0.05 wt% of furfural) but a higher catalyst loading and a longer rxn time could allow
avoiding the autoclave. Nonetheless, the pressure and temperature were relatively mild for an aerobic oxidation.
In theory the inclusion of stoichiometric MnO2 with a Canizzarro drives the rxn to the right:
2RCHO >> RCH2OH + RCO2H
RCH2OH + MnO2 >> RCHO + Mn2O3
This likely explains the rxn above, with Cu as an oxygen activator as it is also used in the Wacker process.
The catalyst is prepared by the thermal decomposition of copper (II) permanganate, which is achieved by autoclaving a solution in the paper, but might
be effectively replaced by isolating the salt.
[Edited on 13-9-2024 by clearly_not_atara]
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi clearly_not_atara; this is an interesting find psyllium ground seeds are as you say readily available. I am coming back to my work on furfural, I
have been somewhat distracted by other things. So I will probably give it a go in the near future. Have you tried working with this stuff? I can
imagine that it will form a gloopy mess to start with. Do you think it would be best to hydrolyse it first with say 1-2% acid first and then add more
HCl and salt for the dehydration of the pentoses?
I wonder if you could isolate the xylose and arabinose from the hydrolysate?
Added comment: have just spotted xylose on ebay for under £9 per kg, less than I can buy psyllium for!
[Edited on 15-9-2024 by Boffis]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by Boffis  | | Hi clearly_not_atara; this is an interesting find psyllium ground seeds are as you say readily available. I am coming back to my work on furfural, I
have been somewhat distracted by other things. So I will probably give it a go in the near future. Have you tried working with this stuff? I can
imagine that it will form a gloopy mess to start with. Do you think it would be best to hydrolyse it first with say 1-2% acid first and then add more
HCl and salt for the dehydration of the pentoses? |
No experience with psyllium, as I said, I posted about it as soon as I found out. I agree it would probably be gloopy. That is its, er, intended
function 
Hydrolysing it first seems like it could help, but it should also hydrolyse in situ. The disadvantage would be in going from the dilute acid to the
strong acid: you either have to add a huge amount of azeotropic HCl, use fuming HCl, or use HCl gas. Neither possibility seems attractive.
Nice find on the xylose. Who would have thought?
|
|
|
| Pages:
1
2
3 |