| Pages:
1
2
3
4 |
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Dehydration AlCl3.6H2O by Thionyl Chloride
   
 
[Edited on 10-9-2013 by Waffles SS]
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Quote: Originally posted by Chainhit222  | I just made anhydrous aluminum chloride. You will need:
-aluminum foil
-a barby
-chlorine or hcl gas source
-a piece of metal pipe (i used copper)
-some way to connect it all together (i afro rigged it)
my setup was:
1) flask connected to a addition funnel (ground glass 24/40)
2) a hose (i used tygon, vinyl is fine) leading from the hose barb on the flask to a ground glass vacuum take off thing wrapped in electrical tape and
wedged into a copper tube filled with aluminum foil from the food store laid across the barby
3) a liebig condenser cooled with room temp water with one end wrapped in electrical tape wedged into the end of the pipe
I preheated the barby as high as it would go (seems to be like 450c) (take off the flame guard if it has one) and then laid the pipe on it. At this
point the pipe was being flooded with argon gas so the aluminum would not oxidize (i dont think it was necessary, but why not if you got a tank).
After I was confident that the pipe and reactants were hot enough I switched the hose from argon to the hcl gas generator and turned it on.
AlCl3 began to sublime and condense in the liebig. Once I ran out of hcl gas I took the liebig off and put it over a container. I then took a clean
plastic glassware brush and pushed/twisted it through the liebig, which caused the alcl3 to fall out into my container. Id recommend a large chlorine
gas generator, I ran out of chlorine so I just got a few grams of AlCl3 which is very slightly contaminated with burnt electrical tape.
If you actually wanna do this, I would recommend adding a drying tube for the chlorine or HCl gas, and using some kind of heat resistant material
(maybe muffler tape?) to make a connection between your glassware and the copper pipe.
http://img807.imageshack.us/img807/241/thugfurnace.jpg (picture of setup)
http://www.youtube.com/watch?v=bpi7cX5d0iw (youtube video of reaction)
Why not use a quarts tube you ask? Because I cannot afford a quarts tube or a tube furnace. I have 12$ in my wallet.
[Edited on 26-7-2010 by Chainhit222]
[Edited on 26-7-2010 by Chainhit222] |
Could gallium be used to amalgamate aluminum and make it more reactive towards chlorine or HCl gas? Gallium makes aluminum react as if it has no
oxide. If gallium is useful in this case, it would possibly make the reaction go quicker and only be a matter of avoiding the use of aluminum tubing
and using proper equipment. 
"Imagination is more important than knowledge" ~Einstein
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Aluminum reacts with HCl on contact, so gallium is not necessary. You just need to keep water out of the mix if you want anhydrous AlCl3.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Aluminum does not react with dry HCl at room temperature.
[Edited on 2-10-2014 by bbartlog]
The less you bet, the more you lose when you win.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Some short notes on an unsuccessful attempt to prepare anhydrous AlCl3, which might save someone the trouble of trying the same thing in
future:
There are some papers that describe the use of methyl sulfonyl methane (dimethyl sulfone) as a solvent for AlCl3, from which solution
aluminum can be plated out. For example: http://www.sciencedirect.com/science/article/pii/S0257897212... . Apparently, AlCl3 is at least somewhat stable in this solvent.
Reading this it occurred to me that it might be possible to dissolve anhydrous CuCl2 in MSM, and then use this solution to dissolve or
react with elemental aluminum, presumably reducing the copper and oxidizing the aluminum to AlCl3, with the solvent also acting as thermal
buffer (this reaction is very exothermic). Although I couldn't find solubility figures for cupric chloride in MSM, it's at least somewhat soluble in a
wide variety of solvents.
Accordingly, I dried some CuCl2 in an oven (grinding in a mortar and redrying once). 6.72g of CuCl2 and 150g of MSM were placed
in a 1L flat-bottomed round flask that had likewise been dried in an oven. With heating on a hotplate, the MSM melted and some (but not all) of the
cupric chloride dissolved. I then added the aluminum, which was in the form of five small millimeter-thick pieces cut from some flashing. This
appeared to be mildly exothermic, as the solution bubbled around and above the aluminum bits.
White crystals sublimed and condensed on to the upper portion of the flask ... anyway, long story short, when I re-melted this the next day, half the
aluminum was unreacted and covered with a black film; the solution had a lot of dark particulate in it, which did not dissolve in dilute (~1%) HCl;
there was a somewhat acrid smell on opening the flask, but not the fuming or HCl stench that I would expect if significant quantities of
AlCl3 were present. Looks rather as if sulfides form (from reduction of the MSM, I suppose) and cripple the reaction.
Anyway, while it might be possible to tweak this somehow (maybe keep it just above the melting point of MSM, or use a co-solvent to make it liquid at
even lower temps), it doesn't look too promising after the first attempt.
The less you bet, the more you lose when you win.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I'm so funking pissed after reading about how it is easy to perform anhydrous AlCl3, while they have no idea about the reaction, and I'm so tired
after spending few days building actual devices and trying different modes, yet my only success was something like 20-30 g of AlCl3 and a lot of HCl
smoke all over the room, because I have no idea how to perform the reaction without massive HCl leakage and AlCl3 hydrolysis having no special
equipment.
I want to pinpoint some problems/mistakes:
- you can't do a straightforward Al+HCl or Al+Cl2 - they won't react at all (anhydrous!);
- and even heating to 100°C is not enough, reaction starts somewhere at 200°C, but optimally is run at 300-800°C;
- the reaction is exothermic, that's why you can actually see the aluminium burning (glowing red) in the HCl/Cl2 flow, if it flows fast enough, this
can make your reactor crack;
- this crap obliterated everything that is colder than 180°C, inlets, outlets, walls, and you condenser is usually a big vessel with flat walls,
which is a bad condenser in fact, that's why the AlCl3 easily makes its way to HCl outlet, clogging it and making you breath a life-given hydrogen
chloride;
- producing 50 g of AlCl3 via HCl method can make a nice hydrogen explosion, destroying the room, until you properly dispose it (fume hood, draw it
outside, burn, catalytically oxidize);
- I see no way to make appreciable amount of AlCl3 without complex heating of reactor part of the system, because you need to keep the very end of the
reactor outlet hot, while condenser right next to it should be cold;
- simax/duran/etc bottle as a condenser is capable of processing not more than 20 g of AlCl3 without disassembing the apparatus (it has polypropylene
blue or polybutyleneterephthalate red ring which are relatively heat-labile);
- you need to pass the HCl/Cl2 through a lot of aluminium, otherwise most of the gas will pass unreacted, because aluminium does not readily reacts
with those gases.
Here's brauer's device that worked for me, until had been clogged.
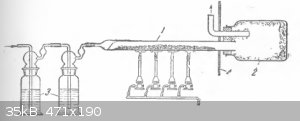
Aluminium amalgam is a known catalyst of Friedel-Crafts, most likely reacting with chlorine in-situ. Maybe some other alloy will work (copper?) or
even a bare aluminium:
"AlCl3 prepd. according to Radzivanovskii [Ber. 28, 1135(1895)] from Al and HCl is an active Friedel-Crafts catalyst.
C6H6 (200 g.) and 4 g. Al shavings treated with dry HCl until a brown coating covered the catalyst, then with 100 g. EtBr, and let stand 48 hrs. at
10-12°, followed by refluxing 2 hrs., gave 73% EtPh, b. 132-4°, d*° 0.8703, nD20 1.4950, 16-18% Et2C6H4" - The activity of aluminum chloride
prepared by the method of Radzivanovskii (benzene alkylation) 000505318-Chem_Abs_45_Friedel_Crafts_with_Al_catalyst.pdf
Also see this thread http://www.sciencemadness.org/talk/viewthread.php?tid=5799
And those guys http://pubs.acs.org/doi/abs/10.1021/ed066p176 claim they've managed to perform Friedel-Crafts with a bare aluminium metal (reaction of secondary
alkyl halide with toluene, aluminium wire activated by polishing it right before addition to the reaction mixture).
Tell me I can't use TFSE and the problems I state are already solved, thus every amatuer chemists can easily make AlCl3 on 1 mole scale.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
My simple preparation of AlCl3: Reduction of anh. ZnCl2 with Al powder:
http://www.sciencemadness.org/talk/viewthread.php?tid=30150&...
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Thanks, you have posted it the second time to me. I mentioned it in my last message. This was a setup I used the first time (brauer's apparatus), and
it partially worked.
|
|
|
SimpleChemist-238
Hazard to Others
  
Posts: 147
Registered: 28-9-2014
Member Is Offline
Mood: Chlorine Trifloride Flame Thrower
|
|
Why not use a long thick glass tube as a reaction vessel and place Aluminium powder into it?
We are chemists , we bring light to the darkness. Knowledge to ignorant, excitement to the depressed and unknowing. we bring crops to broken fields
and water to the desert. Where there is fear we bring curiosity.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
You can't use a thick one - you need to use thin, because of a possible strong overheating (from 200°C to 700-800°C). I was using a tube 1.8 mm
thick, worked okay. Porcelain 5.5 mm thick has thermal shock resistance of 230-280°C http://www.hindawi.com/journals/je/2013/972019/ , while this number is as low as 230°C for my pyrex glass tube (1.8 mm).
I don't think there's much difference in the kind of aluminium I use, turnings work fine, even aluminium wires will work.
I've already explained the problem: it is about exothermic reaction, clogging and long contact time. Contact time is okay in a long glass tube, but
clogging will be a problem, you need to heat the tube all the way to the reciever.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
After some research I came to understanding: if you are an amateur chemist - you don't need the AlCl3. The only reason it is used industrially is to
produce a large amount of something either spending:
- 4000$ for AlCl3;
- 20,000$ for AlBr3;
- 200,000$ for AlI3.
But it is hard to transport the chlorine because it is a gas, so usually some solid carrier is used. NaOCl readily decomposes over time, isocyanurates
and 5,5-dimethylhydantoin cost 10$ per kg, CaCl(OCl) might be a cheap source of chorine, but who cares about those few buckses? For most cases the
minimum is 5$ per 1 kg of Cl2, while cost of bromide salt is 10$/kg.
Using TCCA as a chlorine source, for an amateur chemist the difference is 12$ for 14 moles of AlCl3 and 25$ for 14 moles of AlBr3. Do you really want
to mess with chlorine, to heat the aluminium up to 200-300°C in a continious flow apparatus, while you can just slowly pour a liquid bromine into
aluminium turnings and enjoy a nice sparky and colorfull reaction? Or maybe you want to generate a hell lot of hydrogen trying to perform the AlCl3
synthesis via HCl?
Human can detect as low as 1 ppm of chlorine or bromine in the air, 10 ppm is unbearable, chlorine has IDHL of 10 ppm and 3 ppm for bromine (it's not
LC50, rather highest safe value for prolonged exposure). http://www.cdc.gov/niosh/idlh/7726956.html
I have never had any health problems with chlorine, and once I even had briefly breathed it in - that really hurt my nose and I had a burning
sensation in it for half of a day. So I'd say that bromine handling is completely safe unless you are a douchebag huffing the bromine directly off the
reaction vessel or sitting in a room full of bromine vapor for a few hours.
I'd like to emphasize, that for many purposes AlCl3, AlBr3 and AlI3 are completely interchangeable, you need the same amount of any of these and you
will get the same yields, while some reactions require at least AlBr3 (AlCl3 gives zero yield), and just few can be run on AlI3 only.
For some reason I failed to find similar explanation, and I was sure the AlCl3 is the only thing everybody needs, because almost every book, every
article talks about AlCl3. And AlBr3 is just as well soluble in all the solvent where AlCl3 is soluble, so you just substitute one with another and do
essensially the same reaction.
PS: and AlBr3 actually melts as low as 97.8°C, making it possible to use a regular distillation setup, not the special sublimation device with
complex heating to avoid condensation in a wrong place.
Also, you can make aluminium chloride from aluminium bromide by reaction AlBr3 + CCl4 -> AlCl3 + CBr4.
[Edited on 2-6-2015 by byko3y]
|
|
|
ChemPlayer
Harmless

Posts: 1
Registered: 19-6-2015
Location: Asia
Member Is Offline
Mood: Synthetic
|
|
Anhydrous AlCl3 from ZnCl2
byko3y, I certainly feel your pain (or rather, I did) because a lot of these preparations only seem to work give reasonable yield / purity if you've
got the right sizes of flask, gaps, cooling mechanism, set them up just right etc.
I recently spent quite a lot of time tweaking and have figured out a way of using what equipment i have and can boast a 40% yield of AlCl3 using the
Zinc Chloride and aluminium powder reaction. And it's decent quality - works a treat for F-C acylations and some demethylations too. I reckon 60% is
possible too with some more time.
Video on the process and explanation is here: https://www.youtube.com/watch?v=g7sS69fQMsk
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very nice work! It will be interesting to see if you (or anyone else) can increase yield.
It seems that deposition plates, or a tube, could be used to capture more of the smoke. But how to get this deposit into a bottle without exposure
to humidity would be the trick.
--------------------------------------------------------------------------
Maybe it could be bubbled into an anhydrous solvent like chloroform. Thr chloroform could then be removed by distillation with the vacuum adapter
connected to a moisture guard tube.
[Edited on 20-6-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ChemPlayer  | byko3y, I certainly feel your pain (or rather, I did) because a lot of these preparations only seem to work give reasonable yield / purity if you've
got the right sizes of flask, gaps, cooling mechanism, set them up just right etc.
I recently spent quite a lot of time tweaking and have figured out a way of using what equipment i have and can boast a 40% yield of AlCl3 using the
Zinc Chloride and aluminium powder reaction. And it's decent quality - works a treat for F-C acylations and some demethylations too. I reckon 60% is
possible too with some more time.
Video on the process and explanation is here: https://www.youtube.com/watch?v=g7sS69fQMsk |
Hey! Someone put my work to good use and even provided credit! Excellent, well done and thank you! Not to mention a very clear Utoob, for a change...
I never measured yield so that was useful too. I'm convinced yield can be improved very significantly by using a strong excess of Al
powder. The low yield is in part due to this being a solid-solid reaction: contact between reagents is by definition quite poor.
My roughly stoichiometric ratio was just a starting point, nothing more. Proof of concept.
Losses at the neck could be reduced by slightly lowering external heat once reaction has been initiated: the reaction is just about self-sustaining
(at stoichiometric ratio) and slower evolution of AlCl3 will improve cooling/condensing somewhat. Condensation of the poor heat conductor AlCl3 causes
a temperature gradient to form, with the surface of the freshly condensed product being slightly 'hotter' than the glass itself, reducing heat flow
slightly. 'Slow is more', here I think.
At the very end, the reaction mixture could then be subjected to some extra heat to convert these last bits of ZnCl2.
Quote: Originally posted by Magpie  |
Maybe it could be bubbled into an anhydrous solvent like chloroform. Thr chloroform could then be removed by distillation with the vacuum adapter
connected to a moisture guard tube.
|
Chloroform would have too low a BP for this purpose. The vapours of AlCl3 are at least 100 C, not taking reaction heat into account. Not to mention
potential wetting problems.
[Edited on 20-6-2015 by blogfast25]
|
|
|
ChemPlayer_
Harmless

Posts: 28
Registered: 21-6-2015
Location: Asia
Member Is Offline
Mood: Synthetic
|
|
Thanks for the kind comments 
You've all got me thinking about various improvements now, and I'm itching to try a couple of variations using slower heating, excess Al powder, and
possibly using a quantity of a low bp inert (to AlCl3 and flame anyway) in the receiving container. DCM may be a first choice.
I like the idea that the solvent quickly heating and even boiling off will effectively act as a 'one-shot' rapid efficient heat exchanger for the hot
vapours to condense in the receiver, just so long as it doesn't cause a jet coming out of the container expelling all the product with it!
Thursday is my window of opporunity to test so I will let you know...
(Btw had technical issues with original 'ChemPlayer' user so I had to start a new one 'ChemPlayer_').
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ChemPlayer_
I seem to recall lightly grinding the ingredients together, to improve mixing/reduce ZnCl2 lumps (mine's quite lumpy). Increased homogeneity in
solid/solid reactions can only improve things.
I also wouldn't be too afraid of a little moisture: water will reduce yield somewhat (due to hydrolysis of AlCl3) but nothing else.
Bear in mind that with an excess Al, the reaction front will already run a little cooler because the excess Al will act as a heat sink. Increasing Al
content of the reagent mixture, 'all other things being equal', should increase reaction speed (not really desired, IMO) but mainly increase overall
yield.
I'm convinced that with some tweaking, about 90 % yield should be possible.
But I'd be careful with DCM, if I were you. With such a low BP it's recipe for splattering and hence product losses due to mechanical entrainment.
Thanks again!
[Edited on 21-6-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I also want to complement you on the quality of your UTube presentation.
As you say the ZnCl2 liquifies before the reaction takes off. So this is really a liquid-solid reaction - a great improvement over a solid-solid
reaction.
It seems that at first you are just pushing air out of the adapter and receiving bottle. Then the smoke comes out as most of the air is gone and the
production rate overwhelms the receiving bottle. It's fun to watch!
What fraction of the produced AlCl3 do you think is lost due to escape at the adapter-bottle junction?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
As you say the ZnCl2 liquifies before the reaction takes off. So this is really a liquid-solid reaction - a great improvement over a solid-solid
reaction.
|
It's certainly possible but not 100 % certain, IMO. The external heating is weak, reaction enthalpy low and a gaseous reaction product carries away
quite a bit of enthalpy. I can't say I saw any liquid ZnCl2, obscured perhaps by the fact there is definitely liquid AlCl3 (you can even see it
refluxing in the video). On the other hand, anh. ZnCl2 has a low MP.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by blogfast25  | | The external heating is weak, reaction enthalpy low and a gaseous reaction product carries away quite a bit of enthalpy. |
How can you say that the external heating is weak. Didn't ChemPlayer say that the RBF had to be intensely heated for 45 minutes before the reaction
takes off?
If the AlCl3 is a liquid when formed it looks like it quickly forms a solid in the form of smoke.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
How can you say that the external heating is weak. Didn't ChemPlayer say that the RBF had to be intensely heated for 45 minutes before the reaction
takes off?
If the AlCl3 is a liquid when formed it looks like it quickly forms a solid in the form of smoke. |
4 TO 5 minutes! NOT 45 minutes.
Remember, ChemPlayer got the idea from work I published here and on my own site. Well, in my own two experiments it took about the same time of very
mild heating before the reaction started. My mixture was in a large test tube and I only heated the top end of the material (nearest the condenser).
After reaction started in my case it was more or less self-sustaining (external heating stopped), I did 'poke' it with the Bunsen a couple of times
when it seemed to be slowing down too much. It doesn't take much heat at all to get it going.
The smoke is almost certainly a suspension of solid (already condensed) AlCl3 particles in genuine AlCl3 vapour. If not, it would not slowly waft over
into the condenser but get stuck in the connecting pipe. That happened when in an initial experiment I was using 8 mm OD pipe (very stupidly):
blockage!
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Just out of curiosity, has any one tried to make AlCl3 using anhydrous CuCl2? I know that in solution when you add aluminium to copper chloride it
quickly reduces the copper ions to form copper metal and leaves a clear solution. Could be worth a shot to use a procedure analogous similar to
blogfast's since copper chloride is easy to come by.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
HeYBrO:
The reduction of CuCl2 (anh.) with Al works but it's far more vigorous than for ZnCl2. The reason is the much higher (i.e. less negative) Enthalpy of
Formation of CuCl2 (- 206 kJ/mol, NIST) compared to ZnCl2 (- 415 kJ/mol, Wolfram alpha). As a result, the Reaction Enthalpy is much higher.
The reaction is much more like a fast burning thermite, making the formed AlCl3 harder to capture. Not impossible with some smart engineering
though...
Someone actually did report such an experiment on this forum several years ago. Search for it if you're interested.
[Edited on 22-6-2015 by blogfast25]
|
|
|
ChemPlayer_
Harmless

Posts: 28
Registered: 21-6-2015
Location: Asia
Member Is Offline
Mood: Synthetic
|
|
Yes correct just 4-5 minutes but of moderately strong heating.
What is interesting though (and I'm not sure if you can see it on the video because I only included the last few seconds before the reaction started)
is that the reaction mixture starts off as a solid (ZnCl2 + Al), then seems to turn mostly liquid and bubbles away for a while (possibly some moisture
coming off), but at the point that the reaction starts (and you can see it is at quite a distinct point in time that it 'kicks off') the mixture does
look as though it has become mostly solid again.
Running a stoichoimetic excess of Al is an excellent idea and one I'll try in the next run...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
ChemPlayer_
Don't forget there's some liquid AlCl3 there too: like in my experiment I can see some of it 'refluxing' back into the reactor.
It's all rather hard to see because there's a solid reagent (Al) AND a solid reaction product (Zn).
|
|
|
ChemPlayer_
Harmless

Posts: 28
Registered: 21-6-2015
Location: Asia
Member Is Offline
Mood: Synthetic
|
|
So I sneaked in the time quickly to do another run, this time using 100g of anhydrous zinc chloride and 25g of aluminium powder (~25% molar excess).
I used a 500ml RB flask for the reaction plus the same 29/34 'V' adapter tube, but using a larger 1 litre Duran bottle (with similar cold-pack and
elastic bands for cooling). My objective was to try as hard as possible to minimise losses from the collection bottle to see just how much product
does come over.
Observations:
1. The reaction started faster (~2 minutes) than before.
2. The reaction was slower and steadier, with no 'snow' produced and only puffs of smoke emerging from the collecting bottle during the 2-3 minutes
while the main reaction was taking place (objective accomplished!).
3. I saw the liquid mentioned refluxing back into the flask (which didn't violently react with anything, hence I assume you must be right and this is
AlCl3 or ZnCl3 as opposed to anything aqueous in nature - not that I imagine water can survive long in those conditions anyway).
4. I stopped after about 5 minutes of heating (paranoid about letting atmospheric moisture into the bottle) because there was hardly any more 'smoke'
coming over at this point.
5. The contents of the collecting bottle were much more 'powdery' and less solid adhered to the side of the glass.
6. Empty bottle weighed 565g and with product 598g, so I got 33g of product (~33% yield).
I can only conclude that a lot of the product must be retained in the reaction flask (or the reaction didn't go to completion).
Ok so now I've got the basic set-up nailed (I think) my thoughts on next steps are to see if I can grind up the ZnCl2 into a finer powder (at the risk
of atmospheric moisture absorption however) and hence a better surface area with the Al (and maybe slightly greater excess of Al to temper the likely
faster reaction a bit).
I have to replenish my ZnCl2 stocks first however as this is quite an 'expensive' process unoptimised! I do have a beautiful 100g+ of AlCl3 collected
now though and I may do some more FC experimentation in the interim.
|
|
|
| Pages:
1
2
3
4 |