| Pages:
1
2
3
4
5 |
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Isopropanol purification
Hey,
This might be a little off-topic but I feel it's relevant to aluminium isopropoxide synthesis.
I'm attempting to purify isopropanol. I started off with a blue-dyed cleaning solution, claimed to contain 60-100 % 2-propanol. I filtered it through
activated charcoal and a cotton plug to remove the dye, that was a success. Now, I'm not sure about the water content, but I know it's not anhydrous.
I'm refluxing 250 mL of iPrOH over 30 g dry sodium carbonate right now. Potassium carbonate is normally recommended for drying of alcohols, but I
don't have any. I take it that the sodium salt would do pretty much the same?
Setup: the boiling flask is a 500 mL Kimax round bottom boiling flask. A claisen adapter with a rubber thermometer adapter is attached. Also attached
is a 300 mm Kimax Liebig condenser. On top of that is a 105° distillation adapter used as a drying tube, filled with calcium chloride. The heat
source is a salt water bath heated with an induction stove.
Here are some pictures:



Now, the alcohol tested negative for peroxide with KI + starch, but I like to be better safe than sorry. Vogel recommends SnCl<sub>2</sub>
for removing peroxide. I don't have any of it on hand, but I do have half a kilo FeSO<sub>4</sub>∙7H<sub>2</sub>O. I
suppose it would be just as good as stannous chloride for removing any possible peroxides?
As of now, the alcohol has been refluxing vigorously for an hour. The thermometer is reading just below 81°C, indicating the 91 % iPrOH/water
azeotrope. My plan is to reflux for another hour, filter, add ~5 grams of ferrous sulfate and sodium carbonate, then arrange for downwards
distillation with protection from atmospheric moisture, discarding the last ~50 mL that doesn't come over.
Does that sound sensible?
[Edited on 19-3-2010 by Lambda-Eyde]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I'm pretty sure you will need to go to greater lengths to dry it before it is dry enough to make the isopropoxide. First of all, '60-100%' is such a
big range that it would be nice to narrow it down some before even starting the project; you're talking about the difference between using a little
drying agent and filling the flask full of it. As an alternative to analysis you could just distill over the azeotrope, which would give you a known
amount of water to deal with, before drying and distilling again.
As for the drying agent, I would assume that different drying agents have different partition coefficients that would describe their effectiveness,
with things like sodium metal way way up there and sodium carbonate not nearly so high. Practically speaking, I like to use quantities of drying
agents such that they remain solid at the reflux temperature of my solvent, because I feel like the partition coefficient can't possibly be that high
once the drying agent forms a hydrate melt (this may however be error and confusion on my part). I'm curious whether your Na2CO3 is still solid; the
monohydrate does not melt until 100C, but that only amounts to 6g of water absorbed (!); while the Na2CO3 also forms a decahydrate this is a liquid
hydrate melt at the reflux temperature of IPA, so I would expect it to form a separate liquid phase unless the IPA was quite dry to start with. I
personally dislike both Na2SO4 and Na2CO3 because they need low temperatures to absorb a decent amount of water, and when those conditions are met
they have a tendency to suddenly crystallize into a big hydrate mass that also traps a bunch of whatever it is you're trying to dry. Magnesium sulfate
is a better choice for IPA in my opinion. But even so I suspect you will have to dry multiple times.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
The stuff you bought was probably 91% already. There's no need to reflux it with anything, although refluxing with CaO would remove the remaining
H2O; Na2CO3 will not. If you shake it up with K2CO3 or NaOH, a lower aqueous layer will settle out and can be drawn off. Dry Na2CO3 might work as
well using an extraction like this. The peroxide (that you don't have) is only problematic if you distill it to near dryness. FeSO4 only removes
peroxide in an acidified aqueous medium. SnCl2 is the only thing I know of that will remove peroxides while anhydrous.
There are threads here that describe a proper procedure, which this isn't. I think a paper describing the use of NaOH to get it anhydrous has been
posted. See here
[Edited on 19-3-2010 by entropy51]
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Thanks to both of you for answering.
bbartlog: I wasn't hoping to get absolute isopropanol from this procedure, just a more concentrated than the 91 % solution I (presumably) have.
I am ashamed at my selection of drying agents; I only have sodium carbonate, sodium hydroxide and calcium chloride! When I asked for epsom
salts/bathing salts at the drug store, I was given some hippy crap tainted with dyes and perfumes...
Right now my economy is rather poor, but I will eventually buy K<sub>2</sub>CO<sub>3</sub>, CaO, KOH,
P<sub>4</sub>O<sub>10</sub> among others.
Quote: Originally posted by entropy51  | | The stuff you bought was probably 91% already. There's no need to reflux it with anything, although refluxing with CaO would remove the remaining
H2O; Na2CO3 will not. If you shake it up with K2CO3 or NaOH, a lower aqueous layer will settle out and can be drawn off. Dry Na2CO3 might work as
well using an extraction like this. The peroxide (that you don't have) is only problematic if you distill it to near dryness. FeSO4 only removes
peroxide in an acidified aqueous medium. SnCl2 is the only thing I know of that will remove peroxides while anhydrous. |
Thanks for the advice on reducing agents, I wasn't aware of that. I will have to get some tin and pure HCl.
Quote: Originally posted by entropy51  |
There are threads here that describe a proper procedure, which this isn't. I think a paper describing the use of NaOH to get it anhydrous has been
posted. |
While doing this experiment I found exactly that paper in my chemistry folder. At least I got some valuable lab experience. 
Next, I'll dry it with NaOH according to the method in the mentioned paper and distill.
By the way, is there any easy method for quantitatively analyzing the water content of the alcohol?
[Edited on 19-3-2010 by Lambda-Eyde]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | By the way, is there any easy method for quantitatively analyzing the water content of the alcohol? |
Probably
not. You can probably find a table of specific gravity vs. concentration, but it won't be that sensitive. If it doesn't turn anhydrous CuSO4 blue
it's probably fairly dry. Even if you made it absolutely anhydrous it would absorb H2O from the air.
It probably need not be extremely dry to make isopropoxide. I use gas line anti-freeze straight out of the bottle with no problems. This is
garage chemistry after all.
|
|
|
Reduce-Me
Harmless

Posts: 25
Registered: 11-2-2010
Member Is Offline
Mood: Negative
|
|
Why is the product distilled with vacuum?
|
|
|
maryam
Harmless

Posts: 6
Registered: 14-5-2010
Member Is Offline
Mood: No Mood
|
|
what was the exact T and pressure used in the proceess. I cant get the alcohol distilled and i got only small amount of Al(iso)3 in solid state not
liquid. I dont know Why the product doesnt sit in reciever flask.. vaccum must be so intense! any ideas?
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
This may be slightly off topic, can titanium tetraisopropoxide be synthesized in a similar manner? The standard procedure is the reaction of titanium
(IV) chloride with isopropanol. If this compound could be synthesized in this manner, this would open up many new synthetic options to the advanced
amateur organic chemist. Titanium metal filings are fairly inexpensive, reaction with chlorine would not be very hard to conduct. Possibly, chlorine
could be passed into a suspension of titanium powder in anhydrous 2-propanol, resulting in the formation of the desired titanium tetraisopropoxide.
Amateur NMR spectroscopist
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Doesn't 2-propanol react with chlorine gas in the presence of a Lewis acid? Or does this only apply to HCl + 2-propanol?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Chlorine may oxidise the isopropanol. If you can make the titanium tetrachloride it should be fairly simple, just provide sufficient scrubbing to
catch all that HCl given off when reacting the alcohol.
|
|
|
white rabbit
Harmless

Posts: 33
Registered: 29-11-2009
Member Is Offline
Mood: Groovy
|
|
I have recently duplicated the synthesis as described by Klute. I used 91% IPA. It was combined and shaken with NaOH (10% by weight) and left to
settle out the aqueous layer and then distilled. It was then refluxed and distilled over Na and under nitrogen.
I then used the dry IPA (400 ml) with 1.65g HgCl2, .2g Iodine and 30g aluminum foil to synthesize the Al(O-i-Pr)3. This was done by first removing the
air from the reaction flask and condenser with N2. The pot temp, when refluxing began, was 77*C. I refluxed for about 2 1/2 hours to dissolve all of
the Al. After sitting over night, the IPA was removed by distilling. The still head temp was 78*C. As soon as the still head temp started to decrease
and pot temp began to increase, I removed the condenser and placed the 105* vacuum adapter and a fresh receiver directly to the still head. Vacuum was
then started and gradually increased to 10mm. At 185*C the product was vigorously refluxing on the top portion of the 1liter flask but not coming
over. At this point I completely insulated the top of the flask and the product began to come over in a hurry. Approx 250ml had collected when it
abruptly stopped. I removed the insulation and saw that there was only a dark gray semi solid mass left.
The product was a clear, somewhat refractive, thick liquid, which after 2 days, solidified.
|
|
|
white rabbit
Harmless

Posts: 33
Registered: 29-11-2009
Member Is Offline
Mood: Groovy
|
|
Here are a few pics of the process
removal of excess water by NaOH

Distilling over sodium
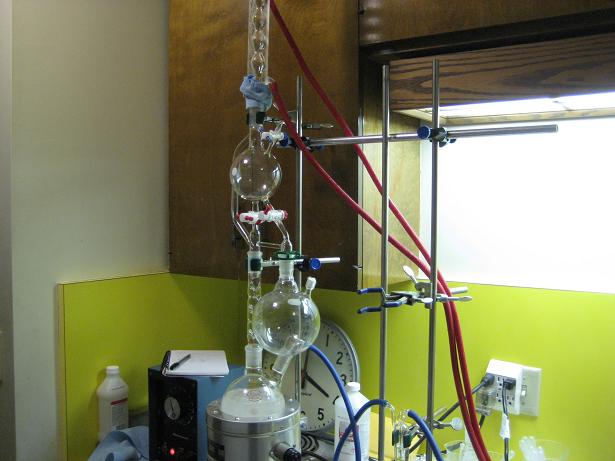
Isopropoxide reaction complete

driving off the excess IPA

distilling over the isopropoxide


final product

|
|
|
maryam
Harmless

Posts: 6
Registered: 14-5-2010
Member Is Offline
Mood: No Mood
|
|
Did you use high vaccum(pump)?(less than 5mm Hg)
|
|
|
maryam
Harmless

Posts: 6
Registered: 14-5-2010
Member Is Offline
Mood: No Mood
|
|
Also Is it enough to use just molecular sieve to dehydrate the alcohol?
|
|
|
white rabbit
Harmless

Posts: 33
Registered: 29-11-2009
Member Is Offline
Mood: Groovy
|
|
The vacuum pump I used only got down to 10mm and molecular sieves with work just fine if you use enough of them.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You shouldn't be pulling a vacuum on flat bottom flasks.
|
|
|
white rabbit
Harmless

Posts: 33
Registered: 29-11-2009
Member Is Offline
Mood: Groovy
|
|
True! But I like the way they sit on the counter when I'm done and that one is really thick.
|
|
|
maryam
Harmless

Posts: 6
Registered: 14-5-2010
Member Is Offline
Mood: No Mood
|
|
my Aluminum starting material is Al metal(Granular) in place of aluminum foil..Is adding iodine important in this case?
|
|
|
maryam
Harmless

Posts: 6
Registered: 14-5-2010
Member Is Offline
Mood: No Mood
|
|
In first step of solid liquid reaction did you take out released H2 gas(purge N2, Use ballon) ? How log the disstilation took? Actually I am
inorganic chemist that s why i have hard time in making it!!thanks for the help..
|
|
|
white rabbit
Harmless

Posts: 33
Registered: 29-11-2009
Member Is Offline
Mood: Groovy
|
|
I just left the N2 flowing about two bubbles per second and a CaCl2 guard at the top of the condenser. The first step took about 4 hours including all
the reflux time. I added the Iodine dissolved in a solution of IPA after the reflux had started. After the addition I noticed an increase in reaction
rate. The second step, under vacuum, only took about 40 minutes.
The granular aluminum will work ,however, the reaction rate may be quite a bit slower because of the lower surface area, (I'm assuming that the
shredded aluminum foil has more). You need to have enough Hg to properly amalgam the aluminum.
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by UnintentionalChaos  | Quote: Originally posted by unome  |
I was wondering if it would be possible, given that the Hg salts are only there to remove the oxide layer, thus allowing the alcohol to come into
contact with the bare metal, if it would be technically possible to utilize a solution of NaOH/iPrOH (which would according the equilibrium be
composed also of NaO-i-Pr and H2O), which would clean the oxide layer off the aluminium, and activate it.
|
That would hinge entirely on whether sodium aluminate is soluble in isopropanol, which I suspect it isn't.
I strongly suspect that gallium salts would work here as a nontoxic alternative, however. A small amount of iodine might also work.
[Edited on 1-25-10 by UnintentionalChaos] |
The insolubility of the aluminate in the isopropanol is the reason I suggested it. It could be removed by filtration, or left behind by distillation.
The reaction with the aluminium would remove the oxide, the clean aluminium would react with the alcohol. There is a similar procedure starting from
aluminium with HCl to give aluminium dithionite in this patent which of course could also be tried (1-2M HCl gas dissolved in anhydrous i-PrOH) would also clean the oxide layer off the aluminium -
forming AlCl3 in situ, or at least a catalytic amount thereof.
Don't know why, but that appeals to me - whether that would be enough to initiate the reaction I could not say for certain.
quam temere in nosmet legem sancimus iniquam
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Could I put small amount of copper chloride to the al-isopropanol mixture to "activate" the al without hg salts? CuCl2 dissolves the aluminium alone.
|
|
|
digitalemu
Harmless

Posts: 38
Registered: 21-10-2010
Member Is Offline
Mood: No Mood
|
|
Just watch it with your own eyes being performed with some background music  Nice work ytmachx... Have some great vids.
Nice work ytmachx... Have some great vids.
http://www.youtube.com/watch?v=axxbIWOge_o
|
|
|
maryam
Harmless

Posts: 6
Registered: 14-5-2010
Member Is Offline
Mood: No Mood
|
|
Anyone knows how to measure purity of aluminum isopropoxide?
|
|
|
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
aluminum isopropoxide prep problem
Using isopropanol, aluminum foil, mercuric chloride and iodine crystals (the latter two in catalytic amounts) I am trying to prepare aluminum
isopropoxide. The reaction seems to proceed but at some point the hydrogen evolution stops.
Can atmospheric water be the problem? I have no idea how sensitive this reaction is to water and in what manner water disturbs it. The IPA is dry to
begin with (sodium was dissolved in it, then it was distilled).
[Edited on 6-8-2011 by Cloner]
|
|
|
| Pages:
1
2
3
4
5 |