| Pages:
1
2
3 |
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I did a few simple experiments with the CS2.
When added to water, it forms a separate layer below the water without any noticeable dissolution.
I added some CS2 to some sulfur in a test tube, swirled it and the sulfur dissolved in a matter of seconds! Surprisingly large amounts of sulfur could
be dissolved in the CS2.
When poured into a shallow dish, the CS2 evaporated, leaving behind some more or less clearly rhombic sulfur crystals.
I poured some of the CS2 into a small beaker, held a metal wire into a bunsen flame for a short period and inserted it into the beaker (it didn't glow
anymore). Whomp! made the beaker and the CS2 burned with a bright blue flame, giving off choking clouds of SO2.
My carbon disulfide has a pleasant aromatic smell, not unpleasant at all. It reminds me of butane for lighters (the kind that doesnt contain
odorants).
I smelled it only briefly, since there's a "toxic" warning label on the bottle and the toxicity on inhalation is stressed in the warnings. I also
conducted the experiments in my fume hood.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Odd, I've always remembered it and described it as having a very unsavory odor that some consider the smell of rotten cabbage, but you say it had a
pleasant aromatic smell?
Merry Christmas by the way.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Is it possible that you had some thiol contamination in your CS<sub>2</sub>? Thiols are generally considered to be very smelly, for
example CH<sub>3</sub>CH<sub>2</sub>SH is listed in the Guinness book as the worst smell in existence.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
That's a definite and probable possibility although it was ACS reagent grade and should not have had such impurities. Well if that is so, then garage
chemist can be proud to have made a better product than Fisher!
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
I'm briefly ressurecting this thread as it's the one with the most suitable title.
Check this out.
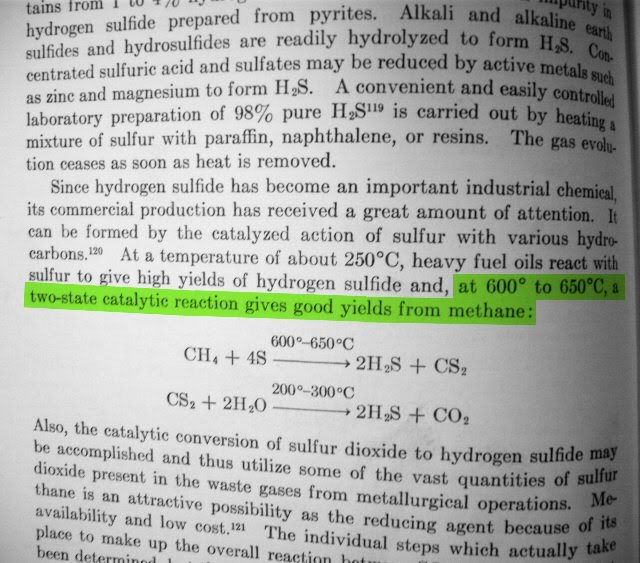
It's about hydrogen sulphide production methods, but there's carbon disulphide, too.
Methane. Cheap and easily available in some areas as natural gas in pipes. The temperature of the synthesis does not pose an unsolvable problem. Has
anyone tried this?
[Edited on 30-5-2012 by Endimion17]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If you look on Lambdasyn (and possibly Versuchschemie too!) you'll see a preparation for schwefelkohlenstoff, a.k.a. carbon disulfide. These guys are
doing it by passing acetylene gas through molten sulfur at about 320-380*C if memory is good. Calcium carbide is cheap and readily available if you
dont have an acetylene cylinder, and this method has the significant advantage that it can be performed in borosilicate glassware (600-650*C will be
too high for this!!).
Links: http://www.lambdasyn.org/synfiles/schwefelkohlenstoff.htm
http://www.versuchschemie.de/topic,12871,-Schwefelkohlenstof...
p.s. the latter is by our very own GarageChemist! And the former may be also...
[Edited on 30-5-2012 by DJF90]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Calcium carbide unfortunatelly isn't readily available, and neither is cheap. It used to be available just about everywhere because it was used for
welding, but today it's quite hard to find. The more one country is developed, the less chance is that CaC2 is lying around cheap. It's simply not
used extensively as in the past.
I found the info on methane in one old book, so I placed it here for the sake of accumulation of information.
Regarding the borosilicate glass, there are plenty reactions done in ceramic tubes and metallic pots. Glassware is for low temperatures, and ~350 °C
is about the highest temperature such glassware was supposed to normally endure without problems.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Yes, I did this synthesis together with a friend some time ago.
The idea came from Sauron in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=12267
The process that Sauron posted works exactly as advertised.
I found that the temperature must be very close to the boiling point of sulfur for the reaction to occur. 430-440°C were necessary for all the
acetylene to react. At less than 420°C, much acetylene passes through the sulfur unchanged. The offgas must be checked often for its acetylene
content.
A thermocouple temperature sensor inside a glass tube must be used inside the reaction, otherwise there's no control over the temperature, and yield
may drop to zero because the reaction is just a little too cold.
Protective gas must be used to flush all air out of the apparatus before beginning to heat the sulfur, because otherwise the CS2 vapor will autoignite
and explode, possibly breaking the reaction flask and scattering molten burning sulfur. I used propane as protective gas because I had nothing else
back then. It was quite a dangerous synthesis and I wouldn't like to do it again.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by Endimion17  | Calcium carbide unfortunatelly isn't readily available, and neither is cheap. It used to be available just about everywhere because it was used for
welding, but today it's quite hard to find. The more one country is developed, the less chance is that CaC2 is lying around cheap. It's simply not
used extensively as in the past.
I found the info on methane in one old book, so I placed it here for the sake of accumulation of information.
Regarding the borosilicate glass, there are plenty reactions done in ceramic tubes and metallic pots. Glassware is for low temperatures, and ~350 °C
is about the highest temperature such glassware was supposed to normally endure without problems. |
The most common use of calcium carbide was in lamps. Bicycle, motocycle, car and hand lamps all used carbide as a fuel source.
Even in the seventies you could still buy it in 1lb tins in cycle shops in Britain.
The best place to get it today is in caving shops.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by ScienceSquirrel  | The most common use of calcium carbide was in lamps. Bicycle, motocycle, car and hand lamps all used carbide as a fuel source.
Even in the seventies you could still buy it in 1lb tins in cycle shops in Britain.
The best place to get it today is in caving shops. |
We still often use carbide for caving purposes because it liberates more heat, which can come very handy in the cold depths, but there aren't
specialized shops. People have their "dealers". 
I know only one store that sells it legally, and that's one pyrotechnic store which sells it for a lot of money. The purpose of it is to create
detonations in barrels for fun. Wikipedia mentions carbide shooting in Netherlands, as if it's their national phenomenon, but it's something people do
pretty much everywhere in Europe, or at least they were doing it when carbide was cheap and readily available.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
There was at least one shop in the UK that used to sell it to cavers until quite recently. I tried to buy some from them but they would only ship to
the UK mainland.
Maybe it has since slipped further off the market.
I bought a jar some time ago from eBay and I only use a few grams at a time so it may outlast me.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
These people will sell it to you in the mainland UK;
http://www.caving-supplies.co.uk/cgi-bin/psProdDet.cgi/26012...
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Endimion17 the first page details some of my first research on using low weight hydrocarbons as a carbon source, what I had found was propane was used
industrially. Due to the ease of dispensing propane at a lab scale vs. methane (without special hooksups) it called to me however it never got past
the drawing board due to the large volume of H2S that would be produced.
|
|
|
| Pages:
1
2
3 |