| Pages:
1
..
24
25
26
27
28
..
45 |
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Teflon begins to decompose @ 260*C and melts @ ~327*C; you'll find you'll have teflon flu symptoms from the decomposition products. . .
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Thanx for the info, I once tried to decompose teflon to its monomer, but even at red heat temps I could not succeeded. Next time I am going to attach
a pt100 temp sensor to the lamp head, I am also interested to know its temp during operation.
[Edited on 6-7-2010 by Jimmymajesty]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Teflon is an idea. I was just wondering about DIY'ing my own HF distillation setup using some.
I would worry about using PTFE around such a hot element however. It would need to be a decent distance from the walls to avoid melting them.
I just did some quick searching and found one place doing 1.5" OD, 1" ID extruded virgin teflon tubing, it's $47.43 per foot ($284.58 for 6). The
molded virgin teflon tubing of 1.5"OD has an ID of 1.25" and is $34.08 a foot ($204.48).
The extruded tubes go up to 3" OD
...OD......ID......code......per foot
1.500 1.250 KM-1116 $34.08
1.750 1.375 KM-1117 $55.42
1.875 1.500 KM-1118 $59.30
2.000 1.500 KM-1119 $81.80
2.250 1.500 KM-1120 $125.74
2.500 2.000 KM-1121 $107.24
3.000 2.000 KM-1122 $234.40
They also do a recycled version of teflon tubing. For a 1.5" OD 1.25" ID tube it's $21.76 per foot ($130.56 for 6).
However, a foot length rod of 1.5" virgin rod is $25.40 ($152.40 for six). Which actually makes it almost as cheap as the recycled, molded stuff, and
you have the option of leaving ends intact, varying the wall thickness and such. Teflon also cuts like butter, so machining it out is not a problem.
You could clamp the rods and use a spade bit to make you own closed end vessels for less than the tubing.
Having read the threads on the ketene furnaces, I'm also not use how productive the lamp is going to be. It might be a better idea to work on
producing a mini, high temperature, cheap furnace.
I was thinking something much smaller than those linear tube furnaces could be make by spiraling some quartz tubing into a coil - rather than having
it as a long rod. I've already found silicon carbide elements that are small and coiled, so they've fit a decent OD tubing inside without being long.
The quartz could sit inside there and be replaceable with different sizes of tube. Or a cup sized crucible for high temperature solid work. Plus
aerogel for insulation. 
[Edited on 6-7-2010 by peach]
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Having read the thread on ketene furnaces was the main cause why I chose the lamp instead of a leaking makeshift furnace
If you can spiral a quartz tubing, then to make a decent ketene lamp would be a joke for ya!
The quartz tube with grounded joints that magpie posted would be my holy grail, it is definitely sg that I cannot afford, to a 1m long tube like that
with ns29/32 grounded joints I would give the ketene lamp and my stock of platinum electrodes too! sorry for being off topic but quartz tubes are my
wet dreams ever since I took up GP catalytic reactions
Literature suggests that with copper tubing the conversion to ketene is almost 100%, cataytic effect is not mentioned IIR, maybe it can be heated more
evenly than any other metal tubes, unfortunately at those high temps, your copper tube turns into black flakes very soon unless you are using reducing
atmosphere.
I can see many flaws with tube furnaces (apart from the lower efficiency, yield, purity and lack of reflux) the main problem is leaking, you cannot
make a reliable connection between metal and glass, even if you can, it will leak after a couple of disassembling.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jimmymajesty  | | you cannot make a reliable connection between metal and glass, even if you can, it will leak after a couple of disassembling. |
Well, you can. They're called Housekeeper seals, after the fellow who invented them. They're easiest with copper, as they rely upon
the flexibility of copper to absorb the difference in thermal expansion rates. They're also not really for beginners, and they usually require a lathe
to turn a fine tapered edge.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
The head of the lamp can be made of teflon. I seal all the glass joints of the lamp, and every glassware with grounded glass joints with teflon tape,
in case of the lamp after 5 runs, the tape was still tape, not a decomposed holed mass.. you can quote me on this
I also distilled H2SO4 at atmospheric pressure at the weekend, and the teflon tape remained teflon tape.. again
Watson there must be a reason that I cannot see home chemists running around with house keeper seals
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Attached image excerpted from
A Dictionary of Applied Chemistry Vol 1 - T.E. Thorpe
Sidebar reference cited , 4 th entry down here _
http://www.lambdasyn.org/upload/acetylchloride-thread/beills...
1111384; Patent; Hoechster Farbw.; DE 210805;
Farbwerke Hoechst Aktiengesellschaft (Formerly) Vormals Meister Lucius Bruning
Patent in German available here _
http://depatisnet.dpma.de/DepatisNet/depatisnet?action=pdf&a...
Click Cancel in the file download window then click - [ Volldokument laden ] - at the upper right
wait a moment , type in requested text ( case sensitive ) click - [ G O ] - to download the full document

[Edited on 5-8-2010 by franklyn]
Attachment: DE000000210805A_all_pages.pdf (112kB)
This file has been downloaded 1127 times
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Thanks, Franklyn, but that German patent DE 210805 appears to be restricted, requiring secure log-in by subscribers to gain access. Please post it
here.
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Franklyn thanx for the info on acetyl sulphuric acid! I made some by leading ketene into sulphuric acid with continuous cooling. It is indeed very
viscous, I poured it into a test tube and it did not even flow out of it when I turned the tube upside down for a moment, it was of honey consistence.
Could one make nitromethane with sulphoacetic acid by sodium nitrite? sorry to be offtopic
[Edited on 4-8-2010 by Jimmymajesty]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
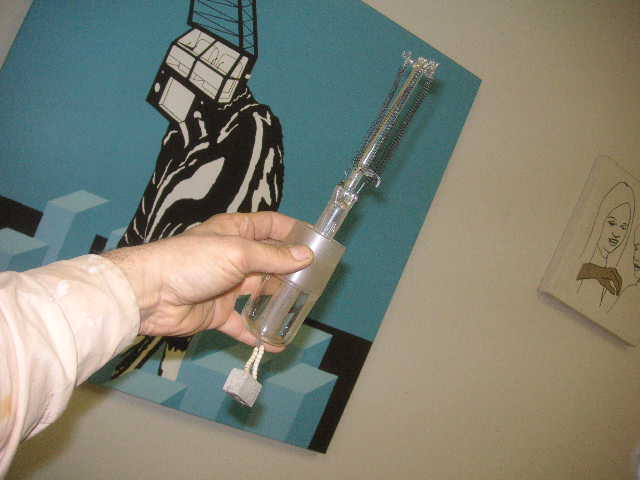
an alternate design requiring one joint, this is more effective imho, hey jimmy whats the second photo in your 'lamp running' doc, of?
|
|
|
Jimmymajesty
Hazard to Others
  
Posts: 153
Registered: 9-7-2009
Member Is Offline
Mood: No Mood
|
|
Hey Panache!
A grounded glass joint to the head of the lamp is necessary to let off the remained air/oxygen before the startup (during reflux), prolonged reflux of
acetone will surely flush all the air off from the head of the lamp, but I would not trust in that. I usually get rid of the remained air by pulling
one electrode off till a condensate starting to wet the grounded glass joint (of the electrode).
The second picture was taken of the cooling element of the ice cube maker after about 4 hours. At normal operation, a small pump fills the tray from a
reservoir, when the small tray is full of water the electronics swith the pump off and the compressor of the cooling circle starts up, but in this
case the small pump and the compressor of the cooling circle runs continously, the small holes on the bottom of tray was made in order to increase the
efficiency of heat exchange.
BTW how did you make the small clamps that holds the filaments apart? I could not really make it out of your pictures could you take some HD photo and
post it in a word doc like I did?
Thx!
P.S I have been thinking for a long time what uses it could have other than generating ketene. I mean, what other usefull chemical could be churn out
by means of this stuff.
I tried to make acetonitrile by boiling ethyl acetate and led dry ammonia into that, then reflux this mixture over standard filament deposited with
fine Al2O3 (sprinkled triethyl aluminium onto the filament) but no luck, the forming acetonitrile clogging the small reflux tubing.
I also tried to deposit SiO2 onto the filament by decomposition of silicium tetraacetate but I failed to deposit a useable SiO2 layer so far.
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Hmmm, glycine, on chlorination in aqueous solution (pH dependent), gives theoretical yields of Cyanogen Chloride (useful bit of information, no?, more
here & here, but it is all over the web if you look).
Cyanogen Bromide, in conjunction with pyridine & acetic acid in benzene (as a diluent/solvent) gives good yields of acetic anhydride (see the
other paper attached).
Anyone got any ideas on how to utilize this without having to isolate the fucking cyanogen chloride? I don't want to breathe it, touch it, or even
stare lovingly at it, I just want from the first to the second flask
Attachment: Ho.Wong.A.Rapid.Method.for.Anhydride.Formation.pdf (150kB)
This file has been downloaded 1185 times
Attachment: Na.Olsen.Mechanism.and.Kinetics.of.Cyanogen.Chloride.Formation.from.the.Chlorination.of.Glycine.pdf (343kB)
This file has been downloaded 1362 times
quam temere in nosmet legem sancimus iniquam
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
The figure on p 1471 of the second pdf you linked does not lead to unbridled optimism about this as a preparative technique. Starting with 10
micromolar glycine they get a concentration of CNCl that tops out around 3 micromolar after 4 minutes, then drops due to ongoing hydrolysis. Seems
like you'd have to remove the CNCl from the reaction somehow, or see whether this reaction can be done under non-aqueous conditions. I think the 13th
reference in this paper (Sawamura, Sakurai &c) might have useful additional information, but I would bet against this as a useful path to acetic
anhydride. Or even cyanogen chloride.
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
As oxidation with chlorine is the accepted process for converting cyanide to cyanate that hydrolysis occurs really isn't a suprise. How to get the
CNCl out of the solution (BP ~12C) and, more to the point (providing the proceeding is viable) dried...
Work that out and there is a chance it could be useful.
quam temere in nosmet legem sancimus iniquam
|
|
|
zed
International Hazard
    
Posts: 2285
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I suggested some time ago, that isopropenyl acetate, if given the opportunity, would actually prefer being transmogrified, into acetic anhydride and
acetone (given the right reaction conditions, and some acetic acid to react with!).
Some may have access to isopropenyl acetate, but not acetic anhydride.
I don't know if this is a viable synthesis, as I haven't seen the full article. Perhaps someone can find a free link to the full article?
Clay Catalysis: A Convenient and Rapid Formation of Anhydride from Carboxylic Acid and Isopropenyl Acetate Under Microwave Irradiation. Synthetic
Communications, Volume 23, Issue 4 February 1993 , pages 419 - 424
http://www.informaworld.com/smpp/content~db=jour~content=a75...
[Edited on 26-8-2010 by zed]
[Edited on 26-8-2010 by zed]
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
IMHO One of the only, truly realistic approaches to (Ac)2O for the amateur chemist remains that of the silica
tetraacetates.
Attachment: Zanetti.Note.on.the.Action.of.Oxychlorides.of.Silicon.on.Sodium.Salts.of.Fatty.Acids.pdf (220kB)
This file has been downloaded 959 times
Attachment: Lanning.Preparation.of.Silicon.Tetrapropionate.pdf (154kB)
This file has been downloaded 1101 times
Attachment: Lanning.Silicon.Tetrabenzoate.pdf (470kB)
This file has been downloaded 1072 times
Attachment: Montonna.SiCl4.as.a.Reagent.for.the.Preparation.of.Acid.Chlorides.pdf (181kB)
This file has been downloaded 1207 times
Attachment: Quig.Wilkinson.Preparation.of.Disilicon.Hexachloride.pdf (303kB)
This file has been downloaded 1085 times
Attachment: Schuyten.Weaver.Reid.Preparation.of.Substituted.Acetoxy.Silanes.pdf (381kB)
This file has been downloaded 1074 times
Attachment: Voronkov.etal.Reaction.of.Carboxylic.Acids.with.Tetrachlorosilane.pdf (154kB)
This file has been downloaded 1534 times
Attachment: Witucki.A.Silane.Primer.Chemistry.and.Applications.of.Alkoxy.Silanes.pdf (113kB)
This file has been downloaded 1073 times
quam temere in nosmet legem sancimus iniquam
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
Better way for making Silicon Tetraacetate
SiO2(s) + 4HF(l) --->SiF4(g) + 2H2O(l)(this reaction need heat)
SiF4(g) + 4NaOAc(s) --->Si(OAc)4(l) + 4NaF(s)(Solvent is anhydrous benzene )
* Beakers and flasks shouldnt made of glass
* SiO2 is available every where
[Edited on 12-9-2010 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Thank you Franklyn for that awesome GERMAN! patent.
This method is so fu***** awesome, why are you guys still looking for other methods?
btw: Franklyn, did you try it?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Regarding this post on Ac2O formation.
http://www.sciencemadness.org/talk/viewthread.php?tid=9&...
@ Jimmymajesty
http://www.sciencemadness.org/talk/viewthread.php?tid=9&...
@ maxidastier
http://www.sciencemadness.org/talk/viewthread.php?tid=9&...
and also from whom I received this U2U _
" hi , I have tried your method for acetic anhydride, via sodium acetate and chlorine
and sulphur. it works pretty well, the only problem was the drying complete of sodium
acetate , ie turning tri hydrate to anhydrous form was a lengthy and messy job. and
then the chlorine leakages are nasty. could you sketch out a nice apparatus from
stainless steel, as glass seems to be difficult for larger quantities. thanks."
- - - - - - - - - - - -
I am not a chemist !
In the course of researching references , when I see a method or procedure which
is of interest to the forum members I post that information faithfully just as I find it.
Polverone said it best " lab work isn't as simple and easy as paper work ! "
I speculated in an earlier thread post that similarly Ca(CH3COO)2 + SO3 might yield Ac2O
http://www.sciencemadness.org/talk/viewthread.php?tid=9&...
but that is just me wondering out loud.
.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
maxidastier: have you tried using a solid chlorinating agent, such as TCCA? Be careful if you do: the rate of gas and heat evolution could be
considerably greater. If this is workable it'd be a lot easier than dealing with gasses.
I believe what's going on here is the formation of S2Cl2, which reacts with 2 mol of sodium acetate to form sodium chloride and a compound of the form
CH3COO-S-S-OOCCH3, which decomposes on heating to yield acetic anhydride, sulfur, and sulfur dioxide. Should be generalizable to other acid anhydrides
as well. You're supposed to use acetic anhydride as a solvent to start the reaction, but it seems that's unnecessary.
I weep at the sight of flaming acetic anhydride.
|
|
|
Luton
Harmless

Posts: 3
Registered: 13-11-2010
Member Is Offline
Mood: No Mood
|
|
Is it possible to use Ethyl Acetate CH3COOCH2CH3 instead of Acetyl Chloride to make Ac20
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Nope.
Also, I should revise my previous idea to carry a harsher warning: it might explode. 
I weep at the sight of flaming acetic anhydride.
|
|
|
Luton
Harmless

Posts: 3
Registered: 13-11-2010
Member Is Offline
Mood: No Mood
|
|
Has anyone successfully tried using Acetyl Chloride with Sodium Acetate to make Ac2O, if you could you please take me step wise 
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
This simple reaction is very well documented - try googling it!
|
|
|
| Pages:
1
..
24
25
26
27
28
..
45 |