| Pages:
1
..
24
25
26
27
28
..
33 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by nitro-genes  | IIRC there seemed to be quite some confusion regarding the formation of either the 2,3,5 or 2,3,6 trinitro 4-aminophenol and corresponding products
upon diazotization like you mentioned a while back. Not sure if a 2,5 dinitro 4-diazo resorcinol would exist and if this would be most likely to form
upon diazotizing the 2,3,5. In this case 2 compounds were wrongly charaterized, which seems very unlikely. If you refer to the compound as DDNR
however, then a 4 diazo would be the same as a 6 diazo right? Can imagine how challenging this must have been in a time where no FTIR/NMR etc was
available to characterize the compounds produced. In addition, many of these nitrations may produce a mix of isomers. Still wonder if this also is the
case for the nitration of paracetamol using conc SA, there is quite some fizzling during the deacetylation, which could originate both from an
N-nitroso compound or 2,3 or perhaps even 2,5 dinitroacetaminophenol. Haha, perhaps the DDNR obtained from the nitration of isopicramic is actually
from further nitration of one of the former derivatives....not likely, but who knows.
Do you have a reference for the nitration of isopicramic? The same reaction at higher temperatures using picramic acid produces mostly DDNP, for
isopicramic I would guess the same. Hmm, even 2,6 dinitro 1,4 phenylenediamine produces pDDNP upon nitration using 100% HNO3/AA, so why the sulfuric
not? Most likely it prevents premature diazotization as pDDNP doesn't nitrate further.
[Edited on 2-2-2017 by nitro-genes] |
Actually classical theory approaches and proofs tend to be quite definitive and conclusive, but requires intelligent, logical application of tests and
proofs. I don't like it when instruments provide readings that contradict classical theory, and in this case I tend to dismiss the instrument readings
and/or the library data or application of such data as suspect. This DDNR compound is obscure enough that it needs good conclusive structure data
verification from different methods to have any confidence in what is being reported. It is particularly troublesome to me that the prior art is not
properly referenced by the modern authors and Klapotke.
References have already been provided and are in this thread back several pages. I would have to go back and review the entire topic, but I am sure
the relevant references are already posted.
Here linked is a post where I was following up on my earlier identification of a probable structure misidentification issue
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
also some explanation here linked
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
Classical nomenclature for phenol would go clockwise with position 1 (X) being the OH of the name compound "phenol" (or similarly 1,3-benzenediol for
resorcinol). But
position 4 (at 6 O'Clock) would be para, and position 6 (at 10 O'Clock) would be ortho, so no position 4 and position 6 are quite different and there
is no "rotation or flipping or reversal or inverting" of the view that accounts for this ring position discrepancy....although there was I think
another different compound from DDNR where a view manipulation might accomplish that explanation.
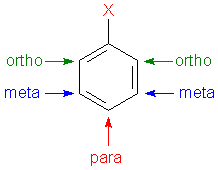
[Edited on 2/3/2017 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Don't know how to read NMR data. The oxide-hydroxide placement maybe matters, so DDNR may not be a good term indeed. Was assuming it would form sort
of one resonance structure, with the metal ion (of the salts obtained) sort of locked in between, I doubt this would really represent 2 different
isolable isoforms, but perhaps not. Only a diazo in 2 would be so probably.
[Edited on 3-2-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A resonance hybrid is something I thought about also as possibly confounding the NMR
Honestly though I think the absence of references by Klapotke to the prior art and history of DDNR is not reassuring, for placing any confidence in
the structural data identifying a 6 diazo, particularly without having a better description for the history and prior art, and explanation of the
justification for the new conflicting NMR data with the experimental data of prior art.
[Edited on 2/3/2017 by Rosco Bodine]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
This is kind of interesting. I was planning on making some p-aminophenol this weekend for a project (I was just planning on making some hydroquinone).
Preliminary research suggests that it's hard to purify due to decomposition.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It is very interesting because a structural data conflict arises with a top notch laboratory that seems to have rushed right past something
significant. I know Thomas Klapotke has some of the references for prior art. I have made contact directly as well as through his grad student
associates.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Something like a charge-transfer complex maybe, like chinhydron?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I need to review all of this thread to make certain neither of us have covered this already, but have you tried subjecting the isopicramic acid to the
reaction condition described by Benedikt and Hubl or Von Herz?
According to B&H the effect of adding a nitro group simultaneously with diazotization is a heat driven reaction "bonus" observed for the
mononitroamidoresorcinol which is IMO an inherently more easily *nitrated* precursor than would be by comparison isopicramic acid. Even so, the
scheme of B&H may follow as a general reaction with some variation applied to similarly effect diazotization and nitration upon precursor
compounds that are susceptible and isopicramic acid might be susceptible, in cold or hot or sequenced reaction, using a nitrate or nitrite.
The acid concentration conditions and reactants of nitrate or nitrite and sequence and temperature could lead to DDNR or to a different or mixed
product result.
Von Herz 1922 patent GB207563 described the boiling condition of SA + NA + an excess of added KNO2 also as a means for pushing the more sluggish and
secondary nitration reaction aspect of the two-step reaction sequence that occurred in situ as a "one pot" diazotization/nitration reaction,
confirming without attribution what was earlier a similar reaction reported by Benedikt and Hubl.
Attachment: Benedikt and Hubl JCS 1881.pdf (264kB)
This file has been downloaded 633 times


[Edited on 2/3/2017 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
They menion dinitroso rescorcinol being converted to styphnic with great ease from even the slightest trace of NA, quite likely a resorcinol is much
more activated than a phenol indeed. In 30% SA, it is most likely that diazotization occurs first iand any nitric formed from the excess nitrite+water
will effect further nitration. The same doesn't apply for isopicramic, which is diazotized and won't nitrate further under condtions that keep it
intact, because the 3 and 5 positions are not activated.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Regarding that diluted H2SO4 described by B&H carelessly, I think they mean to say 26ml H2SO4 + 130ml H2O or perhaps 25ml H2SO4 + 125ml H2O. The
other possibility is 155ml H2SO4 + 775 ml H2O which seems unlikely, as a reaction volume of 930ml to later become filled with reaction product
crystals from 5 grams of precursor. I hate imprecise language in experiment descriptions. That leaves math that doesn't add up and guess work about
what the authors meant to say 
I'm not sure about the deactivation, and have been reviewing what I already checked on the directing effect and I'm sure I wrote about it before.
Yes there is some overlap for the diazotizing and nitrating effect of NO2 that is not entirely selective. And some higher nitrations can occur under
mild conditions from nitroso precursors, actually further oxidized by the same reagent used for "nitrosation".
As an example I think I posted an article describing salicylic acid sulfonate in dilute solution being nitrated all the way to picric acid by gas
dispersion of NO2, and a nitroso intermediate there would likely be a given. The same would likely occur for resorcinol.
Nitric acid is unstable and exists as a sort of equilibrium mixture with nitrous acid, where either one can become the other as needed for a reaction.
I found where you and I were discussing this possible DDNR before with your post linked
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
I have wondered what might be the effect of a sequence of addition for nitrating / diazotizing and cycling temperature might do.
A possibility may exist that a mixed product could be the result where you have p-DDNP "contaminated" with some percentage of DDNR...which might
result in a better initiator "mixture" even though that mixture could be difficult to resolve into its separated components.
In this linked post I described the orienting effect of the 4 diazo on ring nitration for the third entering nitro and provided my opinion then, that
on review I still believe is correct.
There seems to be a good probability that DDNR can result from an isopicramic acid precursor, but identifying the best reaction method there to
accomplish the best yield of DDNR is perplexing. And certainly byproducts of unknown nature are a possibility.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
[Edited on 2/3/2017 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Ah yes, I'm positive the stuff obtained from heating isopicramic with 65% nitric and bicarb neutralization detonated upon heating over flame. The
problem could be that if no pDDNP was left in this case, the ignition temperature could have been much higher and thus more prone to detonate anway.
It is possibe that some DDNR was formed, but also a sodium salt of a triazene, 2,6 dinitro 1,4 benzoquinone and maybe some sodium picrate, depending
on what exactly the light yellow compound is. The puzzling thing is that the triazene seems as energetic upon ignition as pDDNP, whereas I would think
a triazene would behave noticably less energetic. Whatever the light yellow compound is, it is very stable in hot conc NA, SA and nowhere near as
sensitive to light as pDDNP. If it is the triazene, it might be interesting to try and make some nitrate/perchlorate doublesalts. Would a triazene of
dinitro resorcinol also exist? hmm....
[Edited on 3-2-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It is uncertain if the DDNR precursor is actually p-DDNP or is (I think more likely) the diazonium precursor for p-DDNP. It is possible that you
can't get to the DDNR via p-DDNP already formed, but it is the soluble transient intermediate diazonium acid salt precursor for p-DDNP that lies
between isopicramic acid and p-DDNP which is able to add the third entering nitro, which itself immediately decomposes, before the completion of the
diazotization to form DDNR in the alternative to p-DDNP. Under the same conditions both p-DDNP and DDNR could form and it is only which of the two
that is more favored by the reaction conditions that will define the product.
Essentially there is a common diazonium intermediate for both p-DDNP and what is ultimately DDNR, that is an unstable soluble diazonium acid salt that
can go either one of two reaction path ways, to either form p-DDNP, or in the alternative can receive another nitro and ultimately proceed through
other reactions to ultimately form DDNR.
[Edited on 2/3/2017 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Diazo group is meta director just like NO2...now when both are conflicting (in ortho or para) I don't know wich will win...
But regarding hydrolysis (in mild acid, neutral or basic media) the NO2 in ortho is more sensitive because the diazo remains and form a diazo-oxide.
A nitrate or perchlorate of triazene will be hard owing to the very acidic nature of the triazene linked to polynitroaromatic rings...
It is a bit like speaking of nitrotetrazole nitrate or perchlorate...not possible into water...maybe with anhydrous HClO4 and solid
nitrotetrazole...but to my feeling very risky because of the unstability of both reactants.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
So Simple .....isn't it!!!  Ha! this one gives me a headache. Ha! this one gives me a headache.  Brain teaser. Brain teaser.
What I found or dug up a reference for was that a diazo at 4 orients an entering nitro ortho-para which in this case would put the entering nitro at
position 3 or 5 since the para position at 1 is already occupied by a hydroxyl which is nitro substitution inhibiting.
[Edited on 2/3/2017 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
That would be quite novel would it, any ideas what this intermediate would look like, some NOx species involved? So if I would have to make a guess,
you are wondering if nitration of isopicramic using 100% NA would provide a different result than either 65% NA or SA and small quantity of NA? 
[Edited on 3-2-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The soluble diazonium acid salt intermediate is described in the literature including I think some of the articles in this thread that detail the
formation of the diazo oxide. The soluble diazonium acid salt intermediate is only transiently stable in highly acid solution and decomposes on
dilution to precipitate the diazo-oxide. How reversible is that reaction I am not certain. I am not sure that redissolving p-DDNP in acid will
reconstitute the soluble diazonium acid salt intermediate, even though the p-DDNP redissolves.
I am staying with the same analysis and thoughts I shared about this a year and a half ago. I would respectfully submit to scrutiny by my colleagues
in theoretical chemistry, that this particular scenario represents a significant structural "perplexity" arising for the reported NMR data regarding
DDNR and would wish everyone *good luck* with their own analysis. I am feeling a sense of genuine satisfaction that I have identified quite a puzzler
for others' analysis and review / confirmation 
[Edited on 2/4/2017 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by nitro-genes  | That would be quite novel would it, any ideas what this intermediate would look like, some NOx species involved? So if I would have to make a guess,
you are wondering if nitration of isopicramic using 100% NA would provide a different result than either 65% NA or SA and small quantity of NA? 
[Edited on 3-2-2017 by nitro-genes] |
If you review what I posted before linked here, I describe what I still believe is probably correct.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
What are the best conditions is a matter for experiment. I think the methods of Benedikt and Hubl and Von Herz would be good to examine. Maybe look at
all the references and use good parts from each that can be incorporated.
I think there might be benefit from use of a nitrate plus a nitrite.
I am not sure what will be found is the best reaction conditions or what may be the best order of addition and temperature/s. The process details are
purely experimental. What color changes occur could be a way of interpreting what are endpoints where reactions should be considered complete.
It is not necessarily more harsh conditions are the right strategy for introducing a third nitro group, but in fact it may be a niche condition that
does the trick at a specific concentration and temperature, that may be a milder reaction condition than expected. I think that what may occur in
this scenario is similar to what has been observed for nitration of salicylic acid sulfonate all the way to picric acid occurring under mild
conditions and producing quantitative yield. That was so interesting that I posted about it before, linked here. Analogously perhaps m-salicylic acid
sulfonate would provide styphnic acid under mild conditions.
http://www.sciencemadness.org/talk/viewthread.php?tid=4457&a...
When a "niche reaction condition" is favorable for a particular nitration, it will proceed under mild conditions, and this may be the case for the
further nitration observed by Benedikt and Hubl in a similar niche reaction condition scenario that is actually a mild reaction condition
accomplishing what a harsh reaction condition and aggressive use of concentrated acids fails to accomplish.
It would seem possible that the soluble diazonium sulfate intermediate for p-DDNP may operate in conjunction with a sulfonation of the ring at
position 3 forming an intermediate that is easily nitrated at the 3 position. That 3-nitro is unstable and decomposes to a hydroxyl at 3, leading to
DDNR.
[Edited on 2/4/2017 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
It would seem counter intuitive to be able to add a 3-nitro to isopicramic acid by nitration under conditions that would immediately hydrolyse it as
well since the 2,3 nitro presence is not favorable seen the observed ring strain and ease of hydrolysis, in this respect the patents going from
nitroamidorescorsinol at 30% SA and boiling temperatures are noticeably different of course. There are many explanations why isopicramic can seemingly
only form the 4-diazo 2,3,6 trinitrophenol from SA and 2 eqvt of KNO3 . Maybe indeed only some intermediate can be nitrated (nitramine f.e.) further,
although I would think this would be strange considering DNAc can't be nitrated further. It is also possible that oxidation like Philou has shown is
simply competing for the nitric, and 2 mole eqvt's happens to be sort of a coincidental sweetspot for obtaining some of the 4-diazo 2,3,6
trinitrophenol. I must say, I thought usually, 50-65% nitric-water was considered most oxidative and isopicramic is pretty stable up to temperatures
of 60+ C. at these nitric concentrations. Then again, oxidation in 65% nitric may not be comparable in this case, since the water content would lead
to diazotization of part of the product, which would resist any further reaction. Maybe overnitration and sponaneous hydrolysis of a putative
tetranitro are more plausible side reactions competing for nitric (these products would also be oxidized further probably), or further
reactions/stability of any of the products or intermediates in the nitration mix. Maybe the KHSO4 present acts as a solubility modifier allowing
nitration as the free acid, maybe something more like a radical nitration adds the 3 nitro, or catalysis from nitrosylsulfuric present. How soluble is
NO2/N2O4 in sulfuric, does it form some anhydride in there? Seems like a lot of possibilities, and each one and combinations of these would require a
different adjustment of reaction conditions, so I wish anyone good luck trying. 
Maybe lower temperatures, thoroughly dissolving the isopicramic before adding any nitrate and studying the effect of performing the reaction in a
range of 100%-92% sulfuric may help get some more product.
[Edited on 11-2-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
You won't hear me say it is not strange what I have guessed may be occurring. But the sequence for hydroxylation of the ring at 3 necessarily follows
a nitration of the 3 position and that nitration may follow or require a prerequisite sulfonation of position 3 of isopicramic acid. If the
conditions favor hydrolysis of a nitro at 3 then the transition is rapid, but still occurs through the 3 nitro intermediate. I wonder if the 3 nitro
could be introduced in situ following the deacetylation stage by applying the conditions of Benedikt and Hubl, and perhaps DNR would result without
ever isolating isopicramic acid.
The acetyl has to be split off from the DNAc before the other reactions can proceed.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Regarding the diazo at 4 / versus diazo at 6 structural perplexity / anomaly that I have CORRECTLY identified with regards to the classical theory
conflict with currently reported NMR data for DDNR >
Honest actual scientists are here required to acknowledge the obvious conflicting reported NMR data that has been reported.
I have NOT irrationally "imagined" this PERPLEXITY to exist because of impaired discerning or deficiency of critical thinking. I'm 100% sure that I
recognize a very odd scenario and GOOD QUESTION arising in the current "trusted literature" for the diazo compound DDNR identified 136 years ago by
Benedikt and Hubl that EXPLICITLY identifies an unreconciled DEPARTURE for reported NMR instrumental data in direct conflict with established
classical theory.
There is so much "theoretical" contemporary "science" incorrectly proclaimed to be "settled science" about a variety of things that are quite
reasonably still debatable, that I find the irony of identifying this particular DDNR structural anomaly delicious. Indeed I know I am sending some
people "back to school" by my identification of an obvious gap in the science that shows the science is simply NOT settled at all. I have thrown my
colleagues in Munich quite a curve. So you all are invited and welcome to take your best shot at it. I don't really know myself the answer or
explanation for this structural anomaly and perplexity so I can't explain it, other than to point to the anomaly and say it exists.  


|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
At Rosco Bodine,
What was the reaction of the Klapote's team to your indication/error notification?
True that back in those days (one or two centuries ago)...Chemists didn't have as much chemical tools as today and so they were mutlipicating the test
to identify products, specific compounds or mixes of compounds by ingenuous systems/tests...that are rarely used today (*)...
I also think that Klapote's team and other searchers follow the rule of "Publish or Perish" and so quality of the research is sometimes lacking thank
to the frenesy to go too fast in conclusion and yielding some aberrations.
Also there is some lazyness...and probably that they leave programs and algorythms to deliver automated results probably without much
verification...so errors naturally occurs.
(*) I'm translating right now a thesis of 18 pages from French to English related to the isomers of nitration of nitro-anisoles (o, m, p) ...yielding
dinitro-anisoles... The searcher explains in détails how he analyses the various compounds and relative proportions of the various isomers...mp, mp
of mixes with certified isomer synthetized by other routes, solubility in various solvents...preparation of defined proportion mixes and study of the
solubility vs his reaction products...all this without GCMS or H-NMR...also when there is a discrepancy...they go as deep as needed to understand what
happened, and what is the product...
[Edited on 20-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
No response yet on the specifics, not all of which I have shared directly with Klapotke.
Send Klapotke a link to this thread if you or anyone likes. I know he already has the paper by nitro-genes which includes the old references.
Yes, the classical methods of using different routes of synthesis and doing resulting compound identification tests for mixed melting points and mixed
crystallizations, optical properties, solubility tests, known reaction product comparisons as proofs, were usually definitive. I agree that the modern
technology has made modern chemists lazy about more involved classical analysis methods that should receive attention when present method NMR analysis
results disagree with theory. Most of the "old school" chemists who would know best how to perform such classical analytical work and proofs are
probably long retired or departed from this world.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I'm really confused. If the argument is what I think it is...
Klapotke thinks the structure is 6-Diazo-3-hydroxy-2,4-dinitrophenol
aka 6-diazo-2,4-dinitroresorcinol
...and this fits the NMR...
Rosco thinks the structure is 4-diazo-2,6-dinitroresorcinol.
How is this different?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Marvin  | I'm really confused. If the argument is what I think it is...
Klapotke thinks the structure is 6-Diazo-3-hydroxy-2,4-dinitrophenol
aka 6-diazo-2,4-dinitroresorcinol
...and this fits the NMR...
Rosco thinks the structure is 4-diazo-2,6-dinitroresorcinol.
How is this different? |
You don't know? And stil you wrote correctly the two isomers...if they have different names...there is a chance those are two different molecules or
there must be a reason...
A little drawing is better than a long speech so...
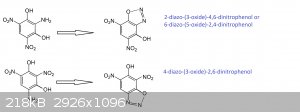
Do you get the difference now?
Are they superimposable by flipping or rotating...?
No --> different compounds.
[Edited on 21-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Rosco also believes that for DDNR the diazo-oxide forms straight across the ring as a para structure, not bonding with the hydroxyl that is ortho,
adjacent as occurs for o-DDNP, but like the structure of the diazo-oxide found with p-DDNP. The tension across the ring bends the entire ring
structure like a hexagonal spring wave washer being bowed by tension across its opposite vertices at para positions 1 and 4, and the diazo-oxide
structure is the bowstring.
[Edited on 2/21/2017 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Rosco Bodine  | Rosco also believes that for DDNR the diazo-oxide forms straight across the ring as a para structure, not bonding with the hydroxyl that is ortho,
adjacent as occurs for o-DDNP, but like the structure of the diazo-oxide found with p-DDNP. The tension across the ring bends the entire ring
structure like a hexagonal spring wave washer being bowed by tension across its opposite vertices at para positions 1 and 4, and the diazo-oxide
structure is the bowstring.
[Edited on 2/21/2017 by Rosco Bodine] |
Simply ask and voilà added 
The structure of trans-diazo is stil a bit mysterious...in reallity it must display a trans quinonoid structure so that both sides can bend onto the
same side of the paper plane while the diazo connects to the opposite side...but connectivity is a bit hard to figure out. Into the book Traité de
chimie organique Azoiques-diazoiques,.... they mention that the probable connectivity passes via a direct link between the N2 and the Cs on both sides
thus without the oxygen being involved...a bit a cage structure related to DABCO(DiAzaBiCycloOctane) but with the Ns at other places and with
unsaturations...anyway this is highly stressed...
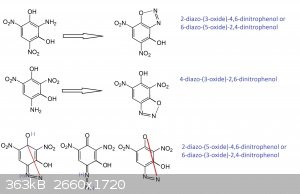
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
24
25
26
27
28
..
33 |