| Pages:
1
..
24
25
26
27
28
..
38 |
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Dr.Bob  | You can heat things in mason jars, just not quickly or with a very hot source of heat. They are made of soda lime glass which expands a lot with
heating, unlike Pyrex or other glasses designed to handle harsh heating well (low coefficient of expansion). But most glasses will break if heated
too fast or unevenly. Round pieces are stronger in general, which is one reason why round flasks are used, not square ones.
If you are trying to modify text tubes, make sure that they are the same type of glass as the tubing that you have. If you have a soda lime tube and
borosilicate glass tubing, they will be very hard to connect together, or for the opposite as well. I see more and more test tubes made of cheaper
non-borosilicate glass nowadays. |
The test tube became deformed at the spot I was applying heat. I think I essentially un-annealed that part of the test tube, and when I moved the
flame to another spot the differential caused it to crack.
Now that you mention it! The glass tube I was attempting to add to the test tube is flint glass! I don't know what difficulty this would add during my
attempts, but this one ended in failure.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Uggh, I was distilling some solvents yesterday outside, and after I was done I went inside and left the apparatus to cool down. I came back around an
hour later and the condenser had shattered. Even though there was salt in the water and the water was still being pumped, the entire jacket froze and
was cracked throughout. Next time I'm adding antifreeze and draining the condenser right afterwards.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's ridiculous! What kind of condenser?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Liebig, it was Chinese and the ice expanded enough to crack it. Don't know if this would have happened with a Pyrex condenser...
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | | Liebig, it was Chinese and the ice expanded enough to crack it. Don't know if this would have happened with a Pyrex condenser...
|
Ice in a Liebig? What happened there?  I mean, unless it was a high MP
solvent...? But the salt was there... Confusing. I mean, unless it was a high MP
solvent...? But the salt was there... Confusing.
I'm thinking of getting a Liebig from a Chinese seller on eBay, costs about $25. Did you have any other problems with yours?
[Edited on 20-1-2015 by Sniffity]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
He didn't put ice in the cooling jacket, he left the cold water in there outside after distillation and it froze. The expanding ice probably would
have done the same in any quality of glass, so I'd say it's better that it happened in a cheap Chinese setup then a Pyrex one.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
No, I didn't have any other problems except for some smaller bubbles around the water outtake of the jacket. All of the condensers had small bubbles
around there, I'm still fighting with the seller about that one. They're telling me in broken English that they will replace them if I have any
issues, anyone else have more experience with this? The seller in question is interlabglassware, anyone know if they could be trusted, or should I
open up a case with eBay? I'm less worried about the condensers, and more worried about the 3-neck 250ml rbf with reasonably big bubbles, a Claisen
adapter with bubbles, and a 100ml addition funnel with bubbles in the neck.
The condenser I got was cheaper, it only cost around $14, but it seemed to work reasonably well.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Too bad.
I have an ancient Pyrex Florence flask of an adult friend of mine's. It has a good sized bubble in the side. It's my most used flask, too.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Years ago, a bright-eyed undergrad, I received my Unknown Compound on the first day of organic lab.
"Put your unknowns in the oven to dry," the supervisor said, "And come to the front for instructions."
Over the next fifteen minutes, paper work we were supposed to have already studied was read aloud to us. "Get to it!" the supervisor said.
I opened the door of the oven to retrieve my unknown. Acrid smoke billowed out of the oven.
Everyone else's white powders were glittering just fine on their filter papers. Mine had decomposed into black goo.
Let's just say that the semester didn't really improve from there. The highlight was the fellow who tried to walk a full pipette of thionyl chloride
from the fume hood to his bench. Vapor pressure quickly built inside, sufficient to empty the pipette all over everything between the hood and the
benches. He tried this twice.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
I've been in the hobby for about 2 years now and I've only had one accident and it was a minor one. I had some 3M Sodium Hydroxide solution in a
glass bottle. Somehow the bottom of the bottle had cracked (probably when I put it down on the shelf last time I used it) and as soon as I opened the
lid it started spraying out everywhere. It went all over most of my labels and I got some on my hands but since it wasn't a very strong solution and
I washed it off pretty fast it didn't do anything besides tingle a little. The biggest casualty was all the labels. The NaOH turned them all green
and made them fall apart.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by gdflp  | No, I didn't have any other problems except for some smaller bubbles around the water outtake of the jacket. All of the condensers had small bubbles
around there, I'm still fighting with the seller about that one. They're telling me in broken English that they will replace them if I have any
issues, anyone else have more experience with this? The seller in question is interlabglassware, anyone know if they could be trusted, or should I
open up a case with eBay? I'm less worried about the condensers, and more worried about the 3-neck 250ml rbf with reasonably big bubbles, a Claisen
adapter with bubbles, and a 100ml addition funnel with bubbles in the neck.
The condenser I got was cheaper, it only cost around $14, but it seemed to work reasonably well. |
Interlab is the sticker on a box set of glassware I bought on Amazon. That sticker was placed over a printed label in Chinese. My point is ALL of the
32 pieces in the set had issues. Mianly air bubbles in the glass, and malformed rims.
Even a product as simple as a glass funnel had a haze to it, and the stem was clearly attached, and not one piece blown/molded.
You will get no satisfaction from them as a company. They wanted me to pay to return (shipping) plus a "re-stock" fee.
I gave the whole deal to my 10yo grandson, and ordered new gear from Deschem on Ebay. I'm not spamming for them... As a business they gave me 10% off
the order, and free shipping over 100.00USD
You can google the company, and find them that way too. They take pride in the glass they produce, and the company is 100% legit.
I'm just trying to help others avoid the Amazon kit, and Interlab.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
Had a little accident doing a synthesis of urine from EtOH with a few ''lab partners''
A champagne bottle slipped through my hand (I had sabered it earlier) and the sharp rim sliced open my hand where the artery enters the index finger.
It soon became a big bloody mess which chased away most of my guests 
I stopped the bleeding and put in 2 stitches but one tore out the next day when I was lifting a coal bag.
This also gave me a chance to look over the first aid kit, which is more of a chest the size of a small travel bag. Looks like most things in there
are past use, tape that doesn't stick, crumbling rubber things, cracked splints, Mercurochrome, chromic catgut sutures (how long have those been
obsolete?),...
we do have a nice collection of hardware though, even a pair of chest cutter (God knows why they are in a first aid kit  ) but apparently my dad used to raid deserted hospitals for the leftover equipment ) but apparently my dad used to raid deserted hospitals for the leftover equipment
On another note: My dad is in Brussels with a brain hemorrhage since yesterday so I have the place to my own
[Edited on 3-2-2015 by Pyro]
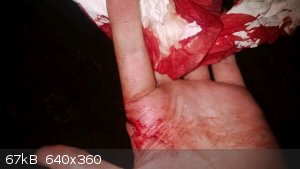
all above information is intellectual property of Pyro.  |
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mayko  |
I opened the door of the oven to retrieve my unknown. Acrid smoke billowed out of the oven.
Everyone else's white powders were glittering just fine on their filter papers. Mine had decomposed into black goo. |
why did that happen ?
| Quote: | | The highlight was the fellow who tried to walk a full pipette of thionyl chloride from the fume hood to his bench. Vapor pressure quickly built
inside, sufficient to empty the pipette all over everything between the hood and the benches |
why couldn't he just use a syringe ? how did the others do it?
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
I set a beaker in the freezer for recrystallization last night, and went to goof around with other things while the solution cooled. It went
completely out of my mind, of course. When I checked today everything was frozen solid; the bottom of the beaker pushed out cleanly, still adhering to
the ice-covered crystals in a nice round fragment. RIP 1000 mL.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Etaoin Shrdlu  | | I set a beaker in the freezer for recrystallization last night, and went to goof around with other things while the solution cooled. It went
completely out of my mind, of course. When I checked today everything was frozen solid; the bottom of the beaker pushed out cleanly, still adhering to
the ice-covered crystals in a nice round fragment. RIP 1000 mL. |
I hear ya. I hate losing my 1000mL beakers.
<sniff>
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by CuReUS  | Quote: Originally posted by mayko  |
I opened the door of the oven to retrieve my unknown. Acrid smoke billowed out of the oven.
Everyone else's white powders were glittering just fine on their filter papers. Mine had decomposed into black goo. |
why did that happen ? |
I would guess that the grad student who was preparing the unknown samples didn't consider thermal decomposition, and the lab supervisor didn't check
the list to make sure they could all be oven-dried safely.
Quote: Originally posted by CuReUS  |
| Quote: | | The highlight was the fellow who tried to walk a full pipette of thionyl chloride from the fume hood to his bench. Vapor pressure quickly built
inside, sufficient to empty the pipette all over everything between the hood and the benches |
why couldn't he just use a syringe ? how did the others do it? |
They were supposed to use the pipette to add the thionyl chloride under the hood, and carry the reaction flask back.
Quote: Originally posted by Etaoin Shrdlu  | | I set a beaker in the freezer for recrystallization last night, and went to goof around with other things while the solution cooled. It went
completely out of my mind, of course. When I checked today everything was frozen solid; the bottom of the beaker pushed out cleanly, still adhering to
the ice-covered crystals in a nice round fragment. RIP 1000 mL. |
My recent adventure in Reagent and Apparatus DeAcquisition:
It's relatively mild, but the wind is howling as I tidy my lab and let a Fischer esterification reflux. The lights go out: at first I think the
breaker has tripped, but in fact the windstorm overhead has taken out the power lines.
Eff it, I say. I unplug my heater and go to sleep early.
I wake up cold. It's really cold outside. I feed the cat, brew some coffee, and stumble outside to restart the reaction, now that the power is back
on.

NO!

NOOOOOOOO
Turns out, water expands upon freezing 
The bright side is, I learned that a pressure-equalizing addition funnel can work as a reflux condenser in a pinch in an unheated lab...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Oh dear. A condenser's much worse to lose than a beaker. Glad you found a substitute though.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's horrible! Sorry for your loss!
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Is there an address to send flowers?
I feel terrible now...
How do you deal w/ Chemical storage? Or the water pipes?
I'd be a nervous wreck all the time.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
| Quote: | | How do you deal w/ Chemical storage? |
Most chemicals contract upon freezing, so although I've watched the acetic acid and the DMSO solidify, the bottles are fine. It hasn't gotten cold
enough to worry about aqueous solutions. (Yay colligative properties!)
| Quote: | | Or the water pipes? |
In my house, yeah... but this is like the one advantage to not having running water in the shed 
| Quote: | | I'd be a nervous wreck all the time. |
Heh... I don't need an excuse for that!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
I smashed my 60mL fritted funnel today  
Went to grab something behind it and as I was pulling it out I bumped it out of the cabinet. Maybe I should rearrange my setup...
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by mayko  | | Quote: | | How do you deal w/ Chemical storage? |
Most chemicals contract upon freezing, so although I've watched the acetic acid and the DMSO solidify, the bottles are fine. It hasn't gotten cold
enough to worry about aqueous solutions. (Yay colligative properties!)
| Quote: | | Or the water pipes? |
In my house, yeah... but this is like the one advantage to not having running water in the shed 
| Quote: | | I'd be a nervous wreck all the time. |
Heh... I don't need an excuse for that! |
Only because I have NO clue on how to make multiple quotes...
I get ya'
One day i'll learn.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Quote: Originally posted by TheAustralianScientist  | I smashed my 60mL fritted funnel today  
Went to grab something behind it and as I was pulling it out I bumped it out of the cabinet. Maybe I should rearrange my setup...
|
Hey TheAustralianScientist~
If you all want a light sucker as a member... I might be interested.
I'm thinking "Black Holes are actually giant drains in the space time fabric.
F" Hawkins, and that other Light bulb guy.
They both have it backwards.
I'll invent the math to prove it (with your groups help)
Bert knows what i'm talking about. So does Mr. BlogFast.
We'll make an EtOH sucker that uses the sun for power to travel to another realm... Oh wait! I already did that.
I'll make the theories, and you guys make the math...
50 /50 but I GET THE WINE!!!!! (45 / 77 ) or something like that.
I KNOW it all sucks, and I can prove it!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
lolwat^^^!
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by Zombie  | Quote: Originally posted by TheAustralianScientist  | I smashed my 60mL fritted funnel today  
Went to grab something behind it and as I was pulling it out I bumped it out of the cabinet. Maybe I should rearrange my setup...
|
Hey TheAustralianScientist~
If you all want a light sucker as a member... I might be interested.
I'm thinking "Black Holes are actually giant drains in the space time fabric.
F" Hawkins, and that other Light bulb guy.
They both have it backwards.
I'll invent the math to prove it (with your groups help)
Bert knows what i'm talking about. So does Mr. BlogFast.
We'll make an EtOH sucker that uses the sun for power to travel to another realm... Oh wait! I already did that.
I'll make the theories, and you guys make the math...
50 /50 but I GET THE WINE!!!!! (45 / 77 ) or something like that.
I KNOW it all sucks, and I can prove it!
|
Zombie, I think you're forgetting your place, you're the Hillbilly. Aga is the Forum Drunkard, only he is entitled to ramble incoherently
|
|
|
| Pages:
1
..
24
25
26
27
28
..
38 |