| Pages:
1
..
23
24
25
26
27
..
33 |
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Has the concept of conjugation and UV absorption due to conjugated bonds been explained yet in this thread, or does that need to be covered as well?
This seems like a good time to do so.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
<b>Conjugation</b>
Conjugation occurs when there are three or more sp<sup>2</sup> or sp hybridized atoms adjacent to each other. Another way of looking at
it is that there must be three adjacent atoms which each contain at least one unhybridized p-orbital. These adjacent p-orbitals tend to overlap as
shown by the following diagram:
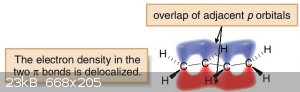
This overlap of p-orbitals allows the pi electrons in the compound to be delocalized and spread across the p-orbitals of all of the conjugated atoms,
rather than being confined to a single atom. Thus, conjugated systems are extremely stable and also exhibit certain special properties. One example
of this is benzene, despite being an alkene, it very rarely behaves like one due to the even spread of electrons throughout the molecule. Unlike the
Kekule structure suggests, all of benzene's bonds are equivalent, not alternating double and single bonds; hence the unexpected behavior. This
unusual behavior is present for most aromatic systems, all of which are conjugated systems.
Conjugated systems also lead to interesting behavior in regards to light absorption. As I believe you know from one of blogfast's lectures(I hope
that it's been explained) atoms can absorb light by promoting electrons to higher, otherwise unused, orbitals. These electrons, which are temporarily
in this higher energy state, are called <b>excited</b> electrons. These excited electrons will then eventually drop back down to their
original lower energy state due to the preferential increase in entropy. The energy which was absorbed thus needs to be released, and this is done
through emitting heat, lower wavelengths of light(such molecules are called fluorescent dyes), or another form of energy. In conjugated systems, the
delocalization of the electrons allows them to be promoted to an excited state by light of a lower energy. Since wavelength is inversely related to
energy, conjugated compounds will absorb light of a higher wavelength than unconjugated compounds. In some highly conjugated compounds, this
wavelength becomes so large it reaches the visible spectrum; thus forming one class of organic dyes.
A molecule can have more than one conjugated system, consider the following example: undeca-1,3,5,8,10-pentaene.
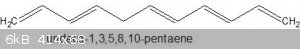
It contains two conjugated systems, one of which has four adjacent carbons with p-orbitals, the other contains six adjacent carbons with p-orbitals.
When determining the wavelength of light which a conjugated compound will absorb, it is based upon the number of adjacent conjugated atoms present in
the largest conjugated system, not the total number of conjugated atoms. Thus a compound such as triphenylmethane, with three smaller isolated
conjugated systems, will absorb light of a lower frequency than anthracene, which contains one large conjugated system.
This concept can be used to explain both the acid-base and variable coloration of phenolphthalein.
Firstly, let's look at the structure of phenolphthalein in neutral or mildly acidic solution. There are three acidic protons, the two on the hydroxyl
groups, and the carboxylic acid(they may or may not be shown as free protons depending on the resonance structure, but they're all there). In a basic
environment, one proton is going to be removed first preferentially, which will be the carboxylic acid. Though both the deprotonated hydroxyl and
carboxylate can delocalize electrons into the aromatic ring, the carboxylate can also delocalize electrons onto the carbonyl, making that proton the
most acidic.
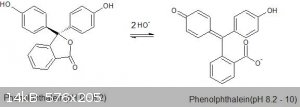
The second deprotonation is where it becomes interesting however. When a proton is removed from one of the hydroxyls, there are two resonance
structures of this di-deprotonated phenolphthalein molecule; both of which are highly conjugated as shown below, connecting all three aromatic rings.
This large number of adjacent conjugated atoms thus lower the energy required to promote electrons enough that phenolphthalein becomes highly colored
in basic solution. Due to the high stability of the conjugated structure, the second proton is quite acidic as well and both deprotonations are
complete at a pH of around 8.2
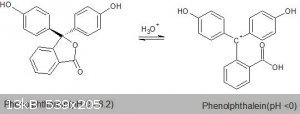
On the other hand, phenolphthalein is also strongly colored at very low pH's, below 0. This is due to the structure of the protonated form of
phenolphthalein, shown below. This structure is also quite highly conjugated, though slightly less so; hence the color being a slightly smaller
wavelength. The key here is that a carbocation is sp<sup>2</sup> hybridized, thus it contributes to a conjugated system as well.
As always, let me know if you have any questions.
Blogfast, please feel free to correct me or expand on any points which I may have missed.
[Edited on 12-4-2015 by gdflp]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@aga
I hope you've been paying attention this semester, because I've written your final exam! The test is 25 questions plus an extra credit question. Just
say the word when you're ready and I'll post it.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Wow, thank you gdflp and Darkstar.
Darkstar, are you aware this forum is MathJax/LaTex enabled?
At the risk of having to move aga into a sensory deprivation tank for a week after this onslaught, here's why pH indicators change colour, in addition
to gdflp's explanation of light absorption by conjugated structures.
pH indicators are weak acids that show different colours depending on whether to molecule is protonated or not, as so completely elucidated by gdflp.
I'll represent the protonated and deprotonated forms resp. as:
$$\mathrm{HIn},\:\mathrm{In^-}$$
In water, the indicator deprotonates weakly according:
$$\mathrm{HIn}+\:\mathrm{H_2O} \leftrightarrow \mathrm{In^-}+ \:\mathrm{H_3O^+}$$
and:
$$K_{HIn}=\frac{C_{H3O}C_{In}}{C_{HIn}}$$
Bear in mind that K<sub>HIn</sub> is really small, typically in the range of 10<sup>-3</sup> to 10<sup>-11</sup>
or so.
We can rearrange the last expression slightly as follows:
$$\frac{K_{HIn}}{C_{H3O}}=\frac{C_{In}}{C_{HIn}}$$
Also remember that:
$$pH=-\log{C_{H3O}}$$
And:
$$pK_{HIn}=-\log{K_{HIn}}$$
We can now see that if:
$$pH=pK_{HIn}$$
... the ratio of protonated to deprotonated indicator equals 1! So if both species have different colours, at that pH the colour will
be in between the colours of the species.
But if:
$$pH < pK_{HIn}$$
... then the protonated species will dominate and the colour will be that of HIn.
And if:
$$pH>pK_{HIn}$$
... then the deprotonated species will dominate and the colour will be that of In<sup>-</sup>.
In summary we can say that:
$$pH=pK_{HIn}$$
... is the turning point of the indicator.
[Edited on 4-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | <b>Conjugation</b>
Conjugation occurs when there are three or more sp<sup>2</sup> or sp hybridized atoms adjacent to each other. Another way of looking at
it is that there must be three adjacent atoms which each contain at least one unhybridized p-orbital. These adjacent p-orbitals tend to overlap as
shown by the following diagram:
|
gdflp, is there perchance a diagram missing?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by gdflp  | <b>Conjugation</b>
Conjugation occurs when there are three or more sp<sup>2</sup> or sp hybridized atoms adjacent to each other. Another way of looking at
it is that there must be three adjacent atoms which each contain at least one unhybridized p-orbital. These adjacent p-orbitals tend to overlap as
shown by the following diagram:
|
gdflp, is there perchance a diagram missing? |
Funny you should mention that, I just noticed that and added it a few minutes ago.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
gdflp:
A point that needs to be stressed with regards to that orbital overlap (aka resonance) is that these π MOs do not in some way "merge", as
that would constitute a gross violation of the Pauli Exclusion Principle, as pointed out higher up in the seminar.
[Edited on 4-12-2015 by blogfast25]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Wouldn't the "Beginnings" forum be a better place for this thread? People interested in the "Chemistry in General" forum are not necessarily
interested in this thread.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
The discussion level of this thread is significantly beyond that of <b>Beginnings</b>. Considering that this started out as a quantum
mechanics thread, the placement in <b>Chemistry in General</b> was entirely appropriate. Now, considering the shift in the subject
matter, it could fit in <b>Organic Chemistry</b> just as well, but there's no reason to move it.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pok  | | Wouldn't the "Beginnings" forum be a better place for this thread? People interested in the "Chemistry in General" forum are not necessarily
interested in this thread. |
I'm not sure how people who are interested in general chemistry cannot be interested in this thread, pok. Chemistry is
quantum chemistry, period.
Also, the beginner's thread is mainly for unreferenced material. And I can't see many total beginners being interested in QM/QC.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | | Now, considering the shift in the subject matter, it could fit in <b>Organic Chemistry</b> just as well, but there's no reason to move it.
|
I've been contemplating asking moderators to splice off the part that follows the main QM/QC thread but like you I can't really see how that improves
things.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Darkstar  | @aga
I hope you've been paying attention this semester, because I've written your final exam! The test is 25 questions plus an extra credit question. Just
say the word when you're ready and I'll post it.
|
Please !
Can't say i'm 'ready' per-se, however fire away.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Pok  | | Wouldn't the "Beginnings" forum be a better place for this thread? People interested in the "Chemistry in General" forum are not necessarily
interested in this thread. |
Beginnings would be fine with me, however In-depth Quantum Mechanics and Organic Chemistry reaction mechanisms don't fit very well in Beginnings.
Given the sheer Range of the material discussed, it is rather 'General Chemistry' in essence.
This thread can simply be ignored by anyone who finds the material of no interest, same as any other thread.
Appologies if this thread pops up to the top of the list too often for your liking.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by gdflp  | Conjugation occurs when there are three or more sp<sup>2</sup> or sp hybridized atoms adjacent to each other.
Another way of looking at it is that there must be three adjacent atoms which each contain at least one unhybridized p-orbital. These adjacent
p-orbitals tend to overlap |
So, essentially the electrons in the bonding orbitals have 'options' on which orbital to occupy next, regardless of which nucleus that orbital is
bound to ?
I can imagine an arrangement where a nearby electron could tip the charge balance causing an electron to 'hop', starting the cycle.
In a molecule like Benzene, if the electrons are truly swapping places continuously, surely that would create some magnetic field effect, albeit tiny.
Is this something that is measurable ?
[Edited on 4-12-2015 by aga]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Yes, the electrons have some freedom to move around in the p-orbitals.
And not that I am aware of. In conjugated systems such as benzene the electrons are free to circulate, but that doesn't necessarily mean that they
flow in one direction. This would create an electrical potential and thus require some input of energy, otherwise it would violate the conservation
of energy. When aromatic rings are placed in strong magnetic fields however, a current is produced, called the <b>aromatic ring current</b>. This current has an effect on certain things, most notably in NMR(Nuclear Magnetic Resonance) spectra.
[Edited on 12-4-2015 by gdflp]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | | I'm not sure how people who are interested in general chemistry cannot be interested in this thread, pok. Chemistry is
quantum chemistry, period. |
Yes. And chemistry is physics. But I personally am not interested in physical chemistry and this thread deals with theoretical aspects.
The "Chemistry in General" forum deals with practical aspects, not theoretical considerations. That's the difference.
@gdflp: If you have a questions-answer thread this is something like a "seminar" and thus indeed belongs to the beginnings. Since there is no
"theoretical/physical chemistry" forum another option would be the "Miscellaneous" forum.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by Pok  |
Yes. And chemistry is physics. But I personally am not interested in physical chemistry and this thread deals with theoretical aspects.
The "Chemistry in General" forum deals with practical aspects, not theoretical considerations. That's the difference.
@gdflp: If you have a questions-answer thread this is something like a "seminar" and thus indeed belongs to the beginnings. Since there is no
"theoretical/physical chemistry" forum another option would be the "Miscellaneous" forum.
|
Why do you believe that <b>Chemistry in General</b> deals with only practical aspects of chemistry? A lack of theoretical topics on this
forum as a whole doesn't mean that they don't have a rightful and important place, despite their rarity. It seems as though you want this topic moved
because you simply lack an interest in it; evidently there are others who feel differently based on the 13,000+ views. You also don't visit this
forum too often, I don't necessarily think that you are as familiar with the placement of topics here. In the end, it's the mods decision and they
have evidently felt that its placement is just fine.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Pok  | ... I personally am not interested in physical chemistry and this thread deals with theoretical aspects.
The "Chemistry in General" forum deals with practical aspects, not theoretical considerations. That's the difference. |
Are the practical aspects of the (currently) failed phenolpthalein synthesis not sufficient to qualify as 'practical' ?
Also gdflp's explanation of the theory behind what Should happen, are they not relevant to the Practical Application of the theory ?
If more Practical Applications and documented reactions using the discussed theories were to be posted (as was My plan, at least) would that bring
this thread back under the Pok radar ?
I smell sour grapes somewhere nearby.
[Edited on 4-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pok  |
The "Chemistry in General" forum deals with practical aspects, not theoretical considerations. That's the difference.
|
Says who? You?
Now please go bore someone else with your pigeon holing. 
There's was always going to be some *diot having to bring up the 'correct placement' of the thread. Sadly, it was you.
| Quote: | | But I personally am not interested in physical chemistry and this thread deals with theoretical aspects. |
Tough shit. Don't read it then.
[Edited on 4-12-2015 by blogfast25]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
@gdflp: I said that this forum (not the topic) deals with practical aspects. I don't think that you have any idea how often I visit the forum. I'm
interested in the "chemistry in general" forum because there are alway new things. But this thread is just a summary of course book, nothing new. I
have to scroll down every time due to the long list of "sticky" topics and this topic here to find the new and interesting topics.
I didn't want to delete this thread but just to get it moved. But okay, I will try to ignore it and scroll down every time like I'm used to ignore
boring pop-ups.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
All this pointless Blart is digressing from the far more interesting subject matter of actual Chemistry, both theoretical and Practical.
Please take all Forum related issues to the "Forum Matters" topic.
Thanks.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pok  | I have to scroll down every time due to the long list of "sticky" topics and this topic here to find the new and interesting topics.
|
You lead a hard life.
You're making a fool of yourself, pok.
| Quote: | | I'm interested in the "chemistry in general" forum because there are alway new things. |
Now you've just made me p*ss myself with laughter: this forum, whatever the section, drowns in duplicates, 'kewls', crap and general flotsam
and jetsam.
[Edited on 4-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
How DARE you !?!?
Not ALL of my posts are utter garbage 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
In a molecule like Benzene, if the electrons are truly swapping places continuously, surely that would create some magnetic field effect, albeit tiny.
Is this something that is measurable ?
|
Remember that electron movement in quantum systems is completely non-Classical.
In the older Bohr model of the hydrogen atom the electron did orbit around the nucleus. One defect that Bohr et al were very aware of was that this
would lead to the electron constantly losing energy (synchrotron radiation) and thus inevitably spiral into the nucleus.
That's, simply put, where Schrodinger/de Broglie came into it: electrons don't move in orbits, they have quantum states. No magnetic field due to
classical orbiting. But quantum systems often do have angular momentum and thus a magnetic dipole. They cause the various forms of macroscopic
magnetism in materials.
[Edited on 4-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So, theoretically, inducing a strong magnetic field in any reaction where a 'ring current' may be present, the reaction could be influenced by the
magnetic field.
I have Sodium Benzoate and some Huge Nd magnets.
Suggestions for a Practical reaction that generally goes One route, and might go another under such circumstances ?
Perhaps a large magnetic field plus electrolysis.
|
|
|
| Pages:
1
..
23
24
25
26
27
..
33 |