| Pages:
1
..
23
24
25
26 |
TheVoid
Harmless

Posts: 10
Registered: 5-1-2018
Member Is Offline
Mood: No Mood
|
|
If your concern is the product not the science the easiest way to benzaldehyde beside buying it directly is reacting benzyl alcohol with bleach and
Hcl acid as seen on youtube, the yields are remarkable if u can make a judgment call on the completion of the reaction. i made an amount of various
aldehydes this way.
|
|
|
Sherlock Holmium
Harmless

Posts: 2
Registered: 15-6-2018
Member Is Offline
|
|
I suggest making benzyl chloride via radical chlorination and a subsequent sommelet reaction to form the benzaldehyde, I imagine this procedure would
give far superior yields, although working with benzyl chloride is not the nicest thing in the world.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Has anyone actually tried the destructive distillation of calcium benzoate and calcium formate under vacuum or inert atmosphere (or it oxidized back
to benzoic acid ).
Would definitely be worth trying.
|
|
|
sykronizer
Harmless

Posts: 18
Registered: 16-12-2017
Member Is Online
Mood: omnivorous
|
|
If you consult more lit on that reaction, you will find that it only works for aliphatic and low molecular weight aldehydes....in any case it is
stated outright that this is not a preparation method.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Could sodium persulfate be substituted with sodium percarbonate in similar context?
|
|
|
TriiodideFrog
Hazard to Others
  
Posts: 108
Registered: 27-9-2020
Member Is Offline
|
|
You can just purchase some bitter almond oil. It is almost pure benzaldehyde.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Quote: Originally posted by draculic acid69  | Has anyone actually tried the destructive distillation of calcium benzoate and calcium formate under vacuum or inert atmosphere (or it oxidized back
to benzoic acid ).
Would definitely be worth trying.
|
Should definitely give a try.
I could make calcium formate instantly at hand stuff.
Calcium benzoate then.. It's not available, but sodium benzoate is.
Sodium Benzoate has solubility of 62-71g/100mL (0-100C).
Calcium Chloride has high solubility.
Calcium Benzoate has solubility of 2-9g/100mL (0-100C).
Na Benzoate + Ca Chloride = Ca Benzoate (s) + NaCl.
So the dry distillation should be performed under vacuum? Does this effect the decomp temp or is it just to protect the expected aldehyde from
oxidizing?
I will put this to my to-do list. When I get time, I'll test it out, unless someone reports here that it's already been debunked.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Persulfate is used in many oxidations for BzH. What is the mechanism in this reaction and could it be substituted with, for example percarbonate or
perborate instead?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Bromobenzene and DMF are not watched chemicals, and can be used to make benzaldehyde by the Bouveault aldehyde synthesis.
there's a limit on how far back the DEA can go in limited precursors. At a certain point it's no longer profitable.
|
|
|
Gualterio_Malatesta
Harmless

Posts: 26
Registered: 8-1-2010
Member Is Offline
Mood: No Mood
|
|
Hey, guys!
There's this paper where they oxidize toluene to BA electrolytically in the presence of NaNO3 and catalytic amounts of
H2SO4.
Toluene (1ml) is mixed with DCM (25ml) then water and sulfuric acid is added (5.4 ml of 37% sulfuric acid diluted with 44.6 ml of distilled water to
obtain 50 ml of 0.46 M solution of sulfuric acid) and 1.18 gm of KNO3. Subsequent stirring of this solution creates an emulsion and current
is applied.
Since I didn't have DCM I used isopropyl alcohol (they also tried MeOH in the paper, so I thought IPA would be ok in this reaction).
The anode (+) was carbon and cathode (-) was stainless steel.
When current (12V, 2.2A) was applied to the reaction mixture (toluene, IPA, KNO3,H2SO4 ) both anode and cathode
produced a lot of gas bubbles (colorless and odorless). The temperature was kept between 30 — 40oC
The smell of toluene completely disappeared after approx 1 hour.
Reaction mixture was filtered from carbon residue to obtain homogenous dark yellow liquid with the smell of IPA and something weird (not BA).
What do you think was the gas from the electrodes and what could have this reaction yielded in the end?
[Edited on 15-12-2020 by Gualterio_Malatesta]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by Gualterio_Malatesta  | Hey, guys!
There's this paper where they oxidize toluene to BA electrolytically in the presence of NaNO3 and catalytic amounts of
H2SO4.
Toluene (1ml) is mixed with DCM (25ml) then water and sulfuric acid is added (5.4 ml of 37% sulfuric acid diluted with 44.6 ml of distilled water to
obtain 50 ml of 0.46 M solution of sulfuric acid) and 1.18 gm of KNO3. Subsequent stirring of this solution creates an emulsion and current
is applied.
Since I didn't have DCM I used isopropyl alcohol (they also tried MeOH in the paper, so I thought IPA would be ok in this reaction).
The anode (+) was carbon and cathode (-) was stainless steel.
When current (12V, 2.2A) was applied to the reaction mixture (toluene, IPA, KNO3,H2SO4 ) both anode and cathode
produced a lot of gas bubbles (colorless and odorless). The temperature was kept between 30 — 40oC
The smell of toluene completely disappeared after approx 1 hour.
Reaction mixture was filtered from carbon residue to obtain homogenous dark yellow liquid with the smell of IPA and something weird (not BA).
What do you think was the gas from the electrodes and what could have this reaction yielded in the end?
[Edited on 15-12-2020 by Gualterio_Malatesta] |
If you are making Nitric acid buffered in situ which is part of what I believe this reaction is doing last thing you want is to use an Alcohol. IPA
might be a bit more stable than some alcohols but no way would I put faith in it.
Maybe... idk, doubtful as well but Ethylacetate? Idk, probably not.
DCM is so easy to get almost everywhere I know of why not just use that? I'm considering this. Don't have any Nitrate though. Have Ammonia Nitrate so
suppose I could just make some. Needed that for Ammonia Generation, if I get around to it I will just save the waste and see about doing this
reaction.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Gualterio_Malatesta
Harmless

Posts: 26
Registered: 8-1-2010
Member Is Offline
Mood: No Mood
|
|
I tried DCM (and the authors mention it too and with good yields), but the reaction temp is 30-40 deg, so DCM basically boils away.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Has anyone thought of the messy one step version where you just dichlorinate toluene to benzal chloride. I guess if you were strategic about it, you
could turn the toluene into benzal chloride by putting the temperature at the boiling point of benzyl chloride. Then, first the toluene goes into the
vapor phase and forms benzyl chloride under UV which promptly condenses. After most of the toluene is gone, the temperature is ramped up with a sand
bath to the boiling point of benzyl chloride, and this undergoes a second chlorination to give benzal chloride. Just add water to get benzaldehyde.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
This isn’t toluene, but phenol (made easily from salicylic acid) can be reacted with chloroform and sodium hydroxide in the Reimer-Tiemann reaction
to form salicylaldehyde. This can be reacted with zinc powder to form benzaldehyde 
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Quote: Originally posted by Opylation  | This isn’t toluene, but phenol (made easily from salicylic acid) can be reacted with chloroform and sodium hydroxide in the Reimer-Tiemann reaction
to form salicylaldehyde. This can be reacted with zinc powder to form benzaldehyde  |
Hmm, I'm right in the middle of making some phenol to mess around with. I have all of those chemicals, I may give this a shot (after I do some
research, of course).
I'll research it myself before I decide if I'll give it a go, but if you have any specific resources/links that you suggest, do share.
Have you tried that synthesis route before?
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I don't have any experience going through the procedure, but the salicylaldehyde synthesis via phenol and chloroform is a standard method. So is the
dehydroxylation of benzene with Zn powder.

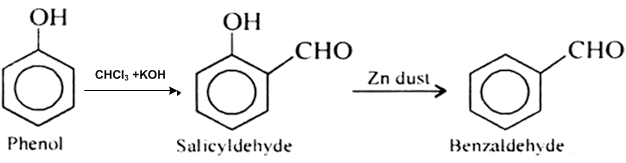
|
|
|
flatstik
Harmless

Posts: 5
Registered: 11-11-2022
Member Is Offline
|
|
Industrial scale in mind...
| Quote: |
The selective liquid-phase oxidation of 3,4,5-trimethoxytoluene to 3,4,5-trimethoxybenzaldehyde, an important chemical intermediate for medicine
production, was developed; when 2.0 mmol of the reactant was heated at 110°C for 2 h in an autoclave under 3 atm O2 with 10 ml of acetic acid in the
presence of 0.75 mmol of Co(OAc)2-Mn(OAc)2 (3:1 mole ratio), a 92% yield of the aldehyde was obtained.
https://sci-hub.se/10.1246/bcsj.61.1035 |
1. Do the metal acetates dissolve in GAA or not?
2. Any idea how the TMBA is extracted from the post rxn mixture? Benzaldehydes generally are soluble in GAA, so extraction with toluene would be the
best option? Also, I'm not particularly interested of solid benzaldehydes, but liquid ones with electron withdrawing groups.
Is the only way to extract with some nonpolar?
It seems (https://www.journal.csj.jp/doi/pdf/10.1246/bcsj.61.967) that they use water to extract the metal acetates -- from the aldehyde is suppose? Do they
then just reuse the GAA-metal salt mixture for next batch?
If this is not the case, recovering the acetic acid is a question mark as well. For example, 13.14mol rxn (2kg of the subst. toluene), 40 liters of
pure acetic acid should be used. Could this be recovered somehow?
The same author has published another method for hydroxybenzaldehydes (https://sci-hub.se/10.1016/0304-5102(92)80027-E). Here no acetic acid is used, but I reckon oxygenating MeOH will yield formic acid in situ?
| Quote: |
Conditions: substrate, 3,5-dimethoxycresol 0.336 g (2 mmol); Ce(AcO)3 0.1 mmol; MeOH 2mL; temp. 110'C; 3 atm O2 in 50 ml autoclave; time, 5 h -- yield
95% |
The extraction protocol:
| Quote: |
Oxygen oxidation was carried out in a 50 ml autoclave equipped with a magnetic stirrer at the desired pressure under constant temperature, using a
methanol solution of the starting materials and catalysts. After a fixed time, the mixture was evaporated to remove the solvents, and the residue was
extracted with EtOAc/HCl in acidic solution (HCl) to remove the catalyst. The EtOAc layer was dried over anhydrous MgSO4 and concentrated by
evaporation |
So, how about this: 1 mol of (un)substituted toluene is heated at 110°C for 2-5 h in an autoclave under 3 atm O2 with 1 L of MeOH per 1 mol of the
substrate) in the presence of Co(OAc)2.4H2O (0.095 times the moles of substrate) of Mn(OAc)2.4H2O (0.28 times the moles of substrate)?
Still, the workup is a question. I'm quite industrial minded and steam distillation is a bitch with volumes I want to work with. I also wonder could
this work with skatole (3-methylindole).
[Edited on 26-11-2022 by flatstik]
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
I heard that pure O2 is not really a forgiving substance when mixed with flammables.
Your suggestion of 110 C, 3 atm O2 and toluene/methanol in a vessel laced with some transition-metal ions (which are generally known as catalysts of
various oxidative degradations of a wide range of compounds) sounds a bit frightening for me. Maybe I'm just a chicken though. 
Honestly, I doubt that you can find anyone on this forum who can answer your questions with certainty. Why not ask the author(s) of the cited
publication?
|
|
|
Waffles SS
Fighter
   
Posts: 999
Registered: 7-12-2009
Member Is Offline
|
|
Catalytic Oxidation of Toluene into Benzaldehyde and Benzyl Alcohol Using Molybdenum-Incorporated Manganese Oxide Nanomaterials
Hamza Shoukat, Ataf Ali Altaf*, Muhammad Hamayun, Shaheed Ullah, Samia Kausar, Muhammad Hamza, Shabbir Muhammad, Amin Badshah, Nasir Rasool, and
Muhammad Imran
Cite this: ACS Omega 2021, 6, 30, 19606–19615
Publication Date:July 20, 2021
https://doi.org/10.1021/acsomega.1c02163
Attachment: acsomega.1c02163.pdf (4.1MB)
This file has been downloaded 385 times
[Edited on 29-11-2022 by Waffles SS]
[Edited on 29-11-2022 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 136
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
The zinc powder is not selective. The reduction of salicylaldehyde running even to benzylalcohol and toluene.
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Waffles SS  | Catalytic Oxidation of Toluene into Benzaldehyde and Benzyl Alcohol Using Molybdenum-Incorporated Manganese Oxide Nanomaterials
Hamza Shoukat, Ataf Ali Altaf*, Muhammad Hamayun, Shaheed Ullah, Samia Kausar, Muhammad Hamza, Shabbir Muhammad, Amin Badshah, Nasir Rasool, and
Muhammad Imran
Cite this: ACS Omega 2021, 6, 30, 19606–19615
Publication Date:July 20, 2021
https://doi.org/10.1021/acsomega.1c02163
|
This is a good read.
Making the catalyst looks like a good project -- remarkably accessible. (I need to check if I have manganese acetate or whether I will need to make
some.)
And the synthesis of benzaldehyde looks reasonable as well. Not great yield at sub-40% and a reaction time of 18h, but pretty straightforward.
This would be worth attempting. Thanks for this, Waffles SS
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Quote: Originally posted by j_sum1  | Quote: Originally posted by Waffles SS  | Catalytic Oxidation of Toluene into Benzaldehyde and Benzyl Alcohol Using Molybdenum-Incorporated Manganese Oxide Nanomaterials
Hamza Shoukat, Ataf Ali Altaf*, Muhammad Hamayun, Shaheed Ullah, Samia Kausar, Muhammad Hamza, Shabbir Muhammad, Amin Badshah, Nasir Rasool, and
Muhammad Imran
Cite this: ACS Omega 2021, 6, 30, 19606–19615
Publication Date:July 20, 2021
https://doi.org/10.1021/acsomega.1c02163
|
This is a good read.
Making the catalyst looks like a good project -- remarkably accessible. (I need to check if I have manganese acetate or whether I will need to make
some.)
And the synthesis of benzaldehyde looks reasonable as well. Not great yield at sub-40% and a reaction time of 18h, but pretty straightforward.
This would be worth attempting. Thanks for this, Waffles SS |
Here ya go, for ease of reference:
Attachment: shoukat2021 - Copy.pdf (4.1MB)
This file has been downloaded 464 times
~Incredibly profound and/or wise quote goes here~
|
|
|
Raid
Hazard to Everyone
  
Posts: 203
Registered: 14-11-2022
Location: N/A
Member Is Offline
|
|
has anyone covered the oxidation of toluene with chromyl chloride?
(Etard Reaction)
[Edited on 31-5-2023 by Raid]
|
|
|
Keras
International Hazard
    
Posts: 1014
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Raid  | has anyone covered the oxidation of toluene with chromyl chloride?
(Etard Reaction)
[Edited on 31-5-2023 by Raid] |
I planned to do that but the idea was superseded by a much less dangerous reaction involving oxidation with cobalt chloride as a catalyser. In my
case, though, I start from cresol, not toluene, and that makes a big difference.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by Raid  | has anyone covered the oxidation of toluene with chromyl chloride?
(Etard Reaction)
[Edited on 31-5-2023 by Raid] |
One page back......
Quote: Originally posted by KiWiki  | Did someone try one of the Drake and Perkin methods:
1. Electrolytic oxidation of toluene, ortho-, meta-, para- xylene in acid solution is a very easy way to prepare the corresponding
benzaldehyde. However, in alkaline solution toluene and meta-xylene will oxidize to carbon dioxide and water.
Source: Trans. Faraday soc., 1905, 1, 31, 251
2. Oxidation with chromyl chloride (see preparation in attachment) to the corresponding benzaldehyde. *
Source: Herbert Drake Law, Frederick Mollwo Perkin, "Oxidation of Hydrocarbons of the benzene series" , J. Chem. Soc. 91, 258, (1907)
*the modern alternative for chromyl chloride might be PCC in Silica gel for this method. I don't have access to these full articles... 
Are there already experimental results that cover these two interestring methods?
EDIT: (by Antrax):
Perform the electrochemical oxidation with 200 to 300 mL acetone to dissolve toluene or xylene. The addition of sodium bisulfite prevents further
oxidation of benzaldehyde. Use 5% to 10% 200 mL H2SO4.
70 A/h /50 g toluene or xyl. Yields are not great as sulphate ions stick to the anode and the fact that benzyl alcohol is formed.
Yields are not higher then 20%, I don't report exact experimental yields and sometimes I even don't give these yields for academic research.
[Edited on 2-1-2018 by KiWiki] |
Quote: Originally posted by sykronizer  | The Toluene of commerce here in NZ and Oz is contaminated with some sulfur compounds, a sulfuric acid wash gives a dark red layer, so what ever method
of oxidation we may try, I strongly suggest a clean up of the Toluene first.
After a few failures I am going to have another go at the Etard reaction, using methylene chloride as the solvent, although it's low boiling point is
not ideal. It is reasonably convenient because the chromyl chloride can be prepared without having to resort to using a retort, for distilling it off.
A word of caution though, do not take short cuts with solvent amounts, ie; make certain you are using at least a 25 percent dilution of the chromyl
chloride to solvent, ditto for the Toluene as well, and constant stirring and cooling. My initial run was a disaster, I was too reckless, and added
the chromyl far too quickly, which resulted in a sudden rise in temperature, that combined with my low boiling point solvent resulted in a stirred
suspension of the adduct going from liquid to solid in about 1 to 2 seconds immediately followed by a mild explosion that covered about 4 square
meters of green lumpy crap , I was uninjured thanks to my safety glasses. |
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
| Pages:
1
..
23
24
25
26 |