| Pages:
1
..
21
22
23
24
25
..
33 |
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
No objections from me, either. I also welcome it.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
If you guys are interested, BKChem and ChemSketch are both freeware molecular drawing programs. The former is what I used before I switched to ChemDraw Pro.
Learning how to quickly draw molecules and mechanisms and then save them as various kinds of images will take a little while at first, but once you
master it, you'll never want to draw them any other way. For instance, in ChemDraw, if you wanted a benzene ring, instead of having to draw it
manually, all you'd have to do is just click the benzene tool on the left toolbar and then click in the drawing space where you want to put it.
Presto! And if you wanted to add a second benzene ring, say one that is fused to the first one (i.e. naphthalene), all you'd have to do is just click again, this time while hovering over one of the bonds on the side of the first benzene ring that
you want the second one to connect to. So in other words, you can literally draw a 10-carbon naphthalene molecule with five double bonds in just three
mouse clicks. Compare that to drawing it in MS Paint-in-the-ass!
Plus it's also nice to be able to just quickly clone molecules by highlighting them and then dragging the cursor away while holding ctrl (as opposed
to manually redrawing them). This is especially useful when drawing mechanisms that involve larger molecules and intermediates (like in the
phenolphthalein mechanism). I know you can clone in MS Paint the same way, but the difference is that you're cloning an image of the
molecule, not the molecule itself. This means everything in the little dotted-line box gets cloned, background included.
Speaking of which, I can tell that blogfast used the clone feature (or copy and paste) in his phenolphthalein mechanism to make phenol. If you look
closely at the phenol molecules, you'll notice that the two carbons on the right side of the benzene ring have tiny perpendicular lines protruding out
from them. My guess is that they used to be part of phthalic anhydride, which he drew first. And then to make phenol, he highlighted the benzene
section of phthalic anhydride and cloned/copied it. 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I got the free ChemSketch program installed now.
Seems relatively easy to use.
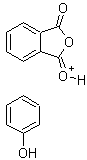
The program stuck the + on all by itself when i added the H.
It was drawn all at right angles, then changed when i clicked on the 'Clean Structure' tool.
I cannot find a tool for drawing curved arrows. Any clues ?
Edit:-
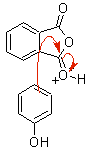
Found it, but it's a bit of a faff.
Click Tools (top menu)
Click Pen Style Panel.
Choose a colour and thickness, then click Apply.
Click Draw (second button from top left, next to Structure)
Click the curved line on the left hand menu (chose how curved)
Click the straight right arrow
Now click on where you want the arrow to start and drag to where you want it to end (It will look silly).
Click the 'select' arrow under the top left Structure button.
Click on your new arrow.
Mess with the resizing dots until it looks as you want it.
[Edited on 30-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Aha. Drawback is that we now have plenty assignments for aga coming up!
Drawback 2 is that I'll have to install it too, to not be left behind.
Darkstar was right about my use of the PITA. I could have covered my tracks better, I guess... The main problem with PITA is drawing the orbital
movements (curly arrows).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I got mine here :
http://www.acdlabs.com/resources/freeware/
gdflp is being quiet, which is ominous, and worrying.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Don't worry, I'm working on the first lecture. Might be another day or two though. Topic is <b>Liquid-Liquid Extractions</b>
ChemSketch is great for drawing structures, I've used it for a couple of years. It's a pain drawing arrows though, I copy the structures if I'm
drawing mechanisms wherever possible.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Just installed mine. Seems very powerful for freeware!
Currently struggling to create a hydroxide ion! You get stuck on the stupidest things when you're new to it...
Good UToob on ChemSketch here:
https://www.youtube.com/watch?v=rRdD95DhPiQ
[Edited on 30-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Excellent video.
I got some ions by clicking on H, clicked in the sketch area and it made H2.
Then click O, and click on the H2 and it makes H2O.
Then click on the + symbol down near the bottom left hand side (actually there's a triangle to select +,-, .+ or .-) then click on the H2O
It automatically makes it into H3O<sup>+</sup> or HO<sup>-</sup>
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Excellent video.
I got some ions by clicking on H, clicked in the sketch area and it made H2.
Then click O, and click on the H2 and it makes H2O.
Then click on the + symbol down near the bottom left hand side (actually there's a triangle to select +,-, .+ or .-) then click on the H2O
It automatically makes it into H3O<sup>+</sup> or HO<sup>-</sup> |
Yeah, just discovered that. It's very intuitive use once you get the basics.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
It can also name compounds by the IUPAC nomenclature, with Tools>Generate>Name for Structure. There's a limit of 50 atoms unless you buy the
full version, but it is still quite a useful tool to check if you're naming compounds correctly as you're learning(or if you learned and forgot ). ).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
ChemSketch is awesome !
One of the best software packages i have ever had.
Believe me, i've had a few ...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
First serious attempt:
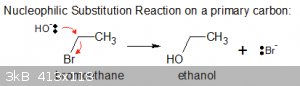
Not too shabby!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Vunderbar !
The ChemSketch people should be congratulating themselves.
The time from Download to producing that image can be measured in mere Minutes.
[Edited on 1-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nucleophilic Substitution Reactions (general case):
In the most general context, a generic case of such a reaction can be written as:
R-Y + :Nu < === > R-Nu + :Y
In that sense it can be regarded as the displacement of a Lewis base (:Y) by a harder one (:Nu).
(Note that if one of the reaction products (or by-products) is volatile or insoluble, a displacement of a harder LB by a softer one may still be
possible due to Le Chatelier)
Below is a simple example, the hydrolysis of bromoethane:
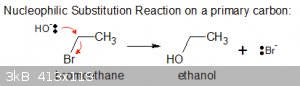
It’s known as an S<sub>N</sub>2 reaction.
But to substitute some Lewis base on a tertiary C atom a different reaction mechanism is called for because steric hindrance prevent the lone pair on
:Nu from accessing the central, tertiary C atom since as it is completely surrounded by methyl groups and :Y itself.
Instead, a carbocation is formed on the tertiary C atom (stabilised by electron pushing), which then joins up with :Nu, as shown
below:
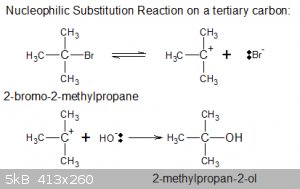
This reaction mechanism is known as S<sub>N</sub>1.
[Edited on 1-12-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Vunderbar !
The ChemSketch people whould be congratulating themselves.
The time from Download to producing that image can be measured in mere Minutes. |
And thanks to ChemSketch, aga can now indulge in his favourite pass time: playing with AOs and MOs:
Structure > Templates > Template window > orbitals

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I mean, who can seriously forget the formation of a full π molecular orbital from two half-filled ungerate p<sub>z</sub> atomic
orbitals, huh? 
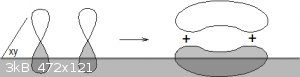
Halcyon days... 
That 3D bit in ChemSketch is also awesome and I don't usually resort to superlatives.
[Edited on 1-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not sure 'awesome' is a superlative, however ChemSketch is Awesome.
Try the 3d modelling.
Edit:
Phenol turned up today, so i can do the phenolpthalein synth.
Would it be better to spin it off into a separate thread or post the method/photygraphs here ?
On the one hand, spinning it off elsewhere would lead to less pollution in this thread.
On the other hand it will add some photos.
Surprising how few participants this thread has attracted.
Perhaps just the Best felt able to get involved.
That can't be right : i'm still here and still fascinated.
[Edited on 1-12-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
I think on this occasion and assuming the synth works more or less as planned, then posting it here as an illustration of a mechanism in
practical conditions would be a Good Thing, pics included.
The 3D bit is awesome. It did get the 3D shape of AlCl<sub>4</sub><sup>-</sup> wrong, yet not
SnCl<sub>6</sub><sup>2-</sup>... Incredible value for no money. 
[Edited on 1-12-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. We get to go Technicolour !
[Edited on 1-12-2015 by aga]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Any thread with "quantum mechanics" in the title is bound to scare a few people away.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yet look at the page view numbers: not bad at all!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Agreed, however the majority ?
Early on there were Others, yet they seemed to Fade pretty fast.
Shame that just a drunkard actually wants to get educated.
Still, an excellent body of Work for Posterity if nothing else.
Soon to have beguiling Photos ...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Synthesis of Alkyl Amines:
An interesting case is the S<sub>N</sub>2 mechanism of the synthesis of alkyl amines, in the example below by reaction of ammonia with
bromoethane:
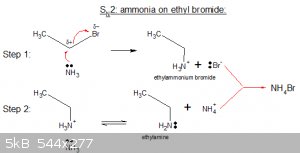
In the first step, a nucleophilic attack by ammonia, a substituted ammonium bromide is formed, in this case ethylammonium bromide (a salt).
Further reaction in step 2 with more ammonia (which snatches a proton, its bonding orbital then folds back onto the N atom) yields ethyl amine and
ammonium bromide as a by-product.
Note that amines (primary, secondary and tertiary, see below) are all still Lewis bases (and in water also Brønsted–Lowry bases) and can thus be
used in further substitution reactions. A tertiary amine reacted with an alkyl halide yields a fully substituted, quaternary ammonium ion (here for
simplicity a quaternary methyl ammonium cation).
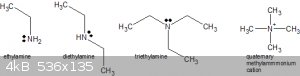
Alkylamines are in fact harder Lewis bases and stronger Brønsted–Lowry bases than ammonia itself (pK<sub>b</sub> for ethylamine is 3.3,
for ammonia 4.75 for instance), due to electron pushing by the alkyl groups.
This reaction mechanism also opens the possibility of creating asymmetric secondary (or tertiary) amines, via:
R<sup>1</sup>-X + 2 :NH<sub>2</sub>-R<sup>2</sup> === >
R<sup>1</sup>-NH-R<sup>2</sup> + [NH<sub>3</sub>R<sup>2</sup>]X
The following SM link links to an active *.pdf on the preparation of ethylamine in 90 % ethanol as solvent.
[Edited on 2-12-2015 by blogfast25]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Liquid-Liquid Extractions
In organic chemistry, extractions are used to separate compounds by exploiting the differences in their solubility in various solvents. There are
varying types of extractions, but only two are commonly used in lab-scale procedures. Liquid-solid extractions can be used to isolate a compound
present in a solid mixture by choosing a solvent such that the desired compound is selectively dissolved. This type of extraction has the distinct
disadvantage that dissolution is a kinetic property, thus extended times and/or harsh conditions are required to ensure a good efficiency.
Liquid-liquid extractions circumvent this by extracting a compound which is already dissolved. The solute merely has to migrate from one solvent to
another, this is a much faster process. In fact, these extractions are quick enough that they can be performed in under a minute per extraction using
a separatory funnel.
Liquid-liquid extractions require two separate solvents, one for the impure solute to be dissolved in initially, and the extraction solvent which
extracts the desired compound from the impure mixture. There is a wide variety of extraction solvents, but there are some characteristics which they
must posses. Firstly, they must be immiscible with the initial solvent, so that the two can be physically separated after the extraction. Secondly,
they must show a good solubility for the compound to be extracted, for example, dichloromethane and diethyl ether are good extraction solvents for
polar compounds, whereas hexane is good extraction solvent for nonpolar compounds. Thirdly, the extraction solvent ideally shows minimal solvation
towards the impurities in the compound to be extracted, to achieve a better separation. Finally, the extraction solvent must have a boiling point
which varies greatly from the compound being extracted, so that the two are easily separated after the extraction. For solids, solvents with low
boiling points are ideally used so that they can be removed quickly and, in the case that the substrate is heat sensitive, the compound doesn't
decompose when the solvent is removed. For obvious reasons, the extraction should not form an azeotrope with the target compound.
The amount of solute which is extracted in one extraction is given by the following equation :Partition coefficient=xvolume of extraction solventamount of solute−xvolume of solution to be extracted where x is
the amount of solute which is extracted in that extraction. The partition coefficient, k, is defined as follows : k=solubility of
compound in extraction solventsolubility of compound in starting solvent k is unique to a solute in a certain set of solvents, and can
either be determined based on reported solubilities or experimentally.
I'm going to define some variables to clean this up a bit : x:Amount of solute extracteds0:Initial amount of solutes:Amount of solute present at the beginning of the current extractionk:Partition coefficientr:Ratio of volume of
extraction solvent to volume of starting solventn:# of Extractions
So, with these new variables we can rewrite the equation above as : Equation 1 : k=xrs0−x=xr(s0−x) This can be rearranged to yield the following equation, solved for x : Equation 2 : x=s0kr1+kr Now let's do an
example to demonstrate the use of this equation :
Assume that the partition coefficient of caffeine in a dichloromethane/water solvent system is 6.4. 200ml of coffee contains 140mg of caffeine. If
the coffee is extracted with 2 x 100ml dichloromethane, how much caffeine remains in the coffee. So, to start off, we plug all of the variables into
Equation 2 : x=.140⋅6.4⋅0.51+6.4⋅0.5=.107g Thus, 107mg of caffeine are extracted in the first extraction. Now we find our new s and
solve for x in the second extraction : s=s0−.107=.033gx=.033⋅6.4⋅0.51+6.4⋅0.5=.025g Finally we add
up the amount of caffeine extracted in the first and second extraction, and subtract from the original amount in the coffee to find the amount of
caffeine remaining in the coffee. .140−(.025+.107)=.008g So, in those two extractions, 94% of the caffeine was removed from the coffee
using 200ml total of dichloromethane. Now let's repeat the problem except this time, we're going to use one 200ml portion of dichloromethane : x=.140⋅6.4⋅11+6.4⋅1=.121g So now, using the same amount of dichloromethane, 19mg of caffeine remain in the coffee,
thus the extraction was only 86% efficient. This demonstrates an important concept in extractions, more extractions using lesser amounts of
solvent are more efficient than fewer extractions using greater amounts of solvent. Of course, this is only the theoretical aspect, in
reality mechanical losses provide a limit to how small the amount of extraction solvent can truly become.
Ignoring that for a moment though, let's look at a way of speeding up determing the amount of solvent extracted in the n<sup>th</sup>
extraction in a series of extractions. Firstly, I am going to define one more constant, b, which will make the following equations more aesthetically
pleasing : b=kr1+kr Now consider an arbitrary number of successive extractions a, the total amount of solute extracted can be
described by the following equation : i=n∑i=1bsi where s<sub>i</sub> is the amount of solute remaining in the
beginning of that extraction. If we expand this to a series, we get : i=n∑i=1bsi=bs0+bs1...+bsn We are given
s<sub>0</sub> in the original problem, but we need to define the rest. We can define s<sub>1</sub> however, in terms of
bs<sub>0</sub> by realizing that s<sub>0</sub> - bs<sub>0</sub> is equal to the solute remaining after the first
extraction which is equal to s<sub>1</sub>. If we make the same argument for the subsequent terms, we get the following : bs0+bs1...+bsa=bs0+b(s0−bs0)+b((s0−bs0)−b(s0−bs0))=bs0+bs0(1−b)+bs0(1−b)2... Thus, the amount of solute extracted in
the n<sup>th</sup> extraction can be defined by : Equation 3 : bs0(1−b)n−1 and the total amount extracted by n
extractions is : Equation 4 : i=n∑i=1bs0(1−b)n−1This can be used to directly compute something quite interesting.
As mentioned previously, a greater number of extractions with smaller amounts of solvent is more efficient than fewer extractions with greater amounts
of solvent, even if the total solvent used is the same. Using the previous definitions, we can adapt the derived equation to represent the efficiency
of a varying number of extractions. If we let : r=rn then we get the following equation : b=kr1+kr=krn1+krn=krn+kr Finally, this can be plugged into Eq. 4. This allows us to calculate and compare the
efficiency of two series of extractions which utilize different numbers of extractions, but the same total amount of solvent.
Just as a note, we can see that : lim thus, theoretically,
as the number of extractions performed goes to infinity, the extraction efficiency approaches 100%(again ignoring mechanical losses).
Edit : Fixed Eq. 2
[Edited on 12-2-2015 by gdflp]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very nice lecture, gdflp. Nice to see there are still some SMers who don't shy away from a bit of math.
What method did you use to render the formulas so well?
Going from Eq.1 to Eq.2, did you lose an index 0 on s?
[Edited on 2-12-2015 by blogfast25]
|
|
|
| Pages:
1
..
21
22
23
24
25
..
33 |