| Pages:
1
..
21
22
23
24
25
..
48 |
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I think you are correct in all your assumptions; a pH probe immersed in an active cell would not only be chemically poisoned, it would be blown out of
the water by stray trons from the process itself. Having messed with pumps and potassium salts, I'll not be trying that again anytime soon, although
a sodium system would be fine. Tap the cell, have a cheap pump trickle the liquor through a section of PVC which carries the pH probe, and make your
measurements.
On the data acquisition front - coming along. I've got a module made up for amplifying a 0 to 100 mV shunt signal to 0 to 5VDC, using an AD626
differential amplifier, so if the hall-effect units don't work out, I can give that one a try. Next in line - plain old cell voltage.
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
wow, i am glad to be back here, my login creds got mixed up for a while and i was just able to recover them...
anyway, about anodes, both of my cells that super cheap mmo held up just as it did for swede. i did get a pretty nice yield from one that i stopped
early. and the next one is still running. i tried drying it in an oven, but i guess it was finished dryin because i tried to ball mill it with a rock
tumbler, but it just made super hard pea sized rocks, some really stragly shape with craters, other just round.
in my second cell i have 9x2 in anodes, both that cheap mmo, running at 14A, compared to my 1.5 by 3.5 @ 12 amps. could the effcientcy change much
from that current density. it seems to be working slower than my first run.
the other differences are that i have a little less saturated solution than last time, and also my electrodes are closer together, so the resistence
is only .25 ohms compared to .6. it runs cooler because of the less wattage, but i may not have enuf voltage, IDK
first cell: 6.3v @ 12A
new cell: 3.5v @ 14A (closer electrodes with more area, seems slower)
those are the best numbers i could remember, my log book is not with me at this moment
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
This may be old and well known, so forgive me if this is redundant.
There is a way to use the audio ADC built into most PCs as a data collection input. Normally this is difficult because (1) the input is AC coupled and
(2) the gain is arbitrary. To get around this, chop the input and multiplex it with a reference signal. This gives a waveform which can be analyzed at
one's leisure.
If anyone is interested I'll post a schematic for such a beast. It isn't very complex and should be cheaper than a dedicated ADC board.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I'm not so sure the current in the cell would necessarily affect the pH probe - the only way to be sure is of course, to try it.. Which I can't do at
the moment - our place is next up for new carpet so I can't setup the cell until I know when that's going to happen - I'm going to need that storage
space it'll take up pretty soon. At any rate, my reasoning for believing there won't be any effect on the probes is because they work fine in
saltwater aquariums - I've seen as much as 12V potential in those, either from pumps/devices in the water, or the chemistry of the tank generating
voltage. Always had a titanium grounding probe in mine. It's surprising to reach into your tank (while grounded) and get a nasty shock!
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
Re tentacles: A static potential probably wouldn't be a problem - the input impedance of the meter is high so as long as the
electrode can float to the same voltage no current will flow. There's no active electrolysis so the redox potential of the solution is close to 0. In
a chlorate cell there's active current passing so an electrode will have a potential gradient across it. That generates a redox gradient which could
cause dramatic chemical activity. In either case, if a path exists from the electrode to the power supply connected to the tank and the input
impedance of the meter is not high enough (especially through the shield/ground or reference connection), current will flow through the electrode and
cause reactions there.
A battery powered pH meter with a very small radius electrode would probably work in an electrolytic cell for a while, perhaps a long while. It
probably would last longer if it was well out of the way of the main current flow. Even so, I don't know how well an Ag/AgCl electrode does with
chlorate ions (haven't looked it up yet, sorry) or how long epoxy encapsulation would hold up in warm acidic chlorate solution (I know this will
eventually destroy it - again, it might take long enough not to matter). The glass part of an electrode would probably be OK.
Anyway, all that is why I'd put my pH probe in a side loop which had very little current passing through it i.e. voltage drop across the loop is close
to 0 and either very well isolated from the equipment ground or with a (sacrificial?) electrode connected to the equipment ground near the probes. And
I'd try to draw the fluid from a place where the electron flow and ion flow mostly canceled each other.
I have to pay for my electrodes, so I baby them  . An old electrode has a good
probability of either being unstable or not working at all so getting used ones is a game of chance. . An old electrode has a good
probability of either being unstable or not working at all so getting used ones is a game of chance.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by densest
I have to pay for my electrodes, so I baby them  . An old electrode has a good
probability of either being unstable or not working at all so getting used ones is a game of chance. . An old electrode has a good
probability of either being unstable or not working at all so getting used ones is a game of chance. |
This is definitely a problem. I refuse to buy used or even new old stock electrodes from eBay, because if they were not properly stored, they may be
worthless, and I think it's more likely they are than not. New electrodes of a decent quality are expensive. The only one we found that was
guaranteed NOT to be vulnerable was over $900!! At a minumum, I'd go with double-junction, and the body needs to be glass or a compatible plastic.
Epoxy in a chlorate cell will not last long, I'm afraid.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by densest
Anyway, all that is why I'd put my pH probe in a side loop which had very little current passing through it i.e. voltage drop across the loop is close
to 0 and either very well isolated from the equipment ground or with a (sacrificial?) electrode connected to the equipment ground near the probes.
|
As long as your circuit is electrically isolated, its internal potential will float to eliminate current
flow. The simplest way (although not the cheapest) is to use an isolation transformer and pay attention to isolating the "ground", here an only-local
ground, on the pH meter side. Sometimes the power supply transformer provides isolation, sometimes not because two legs of the transformer are
"case-grounded" in anticipation of an ordinary safety ground application.
In every case, though, there's the need to maintain a strictly isolated ground. In practice, that means plenty of insulation and no exposed metal
parts (which would require safety grounding, negating the whole point).
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
pH controlled Graphite cell contents
Hello,
Took some photo's of the end products of two Graphite Anode cell runs.
The yellow liquid is from the Na Chlorate run. All the Graphite EVENTUALLY settles out leaving a yellow
liquid. In times gone by, there has been many a discussion as to what the yellow may be. Perhaps this explains
it, perhaps not. You don't seem to get any yellow if you do not run the cell untill the Graphite starts to erode at
an unacceptable rate.
I recrystalized the K Chlorate from the next run. It cleans up OK. The mother liquor as shown too. All the Graphite
settles out OK (no yellow). A few grams of K Chlorate needles appeared too. I filtered all Graphite from the K
Chlorate cell and weighted. It came to a (very small) 1.9 grams for the 422grams Chlorate made, which is very
low. There would still be Graphite in the solid Chlorate but it would only be milligrams.
It is a pitb to process K Chlorate that has been made using Graphite. If making K Chlorate use the Na salt
and convert after solution has been filtered. (as many here have said).
If you are happy with somewhat discolored product than it is fesible to go straight with the Graphite I guess in a pH controlled cell that is.
Dann2
[Edited on 4-3-2009 by dann2]
[Edited on 4-3-2009 by dann2]

|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Nice pics, Dann2. I'm assuming the second pic is chlorate, not chloride... looks like a typo. I think you've shown that decent graphite anodes can
make all the chlorate you'd ever need for either pyrotechnic use, or as feedstock. It's odd, with all the chlorate I've made in the last few months,
I've always gotten the plate crystal form, never the needle. Even when a solution is slowly cooled, or the conditions are otherwise primed for that
form, it never seems to happen. On the contrary, I always end up with pretty massive plate crystals shaped like your second picture.

This was from the 4.4 kg T-Cell harvest, where there was a distinct upper layer, and a much more compact lower layer. I finally figured out what
caused this... the lower layer formed while the cell was hot, and actively producing. It was very firm and compacted, and required hammering to break
up. The upper layer formed when I pulled the plug, took the rig outside, and cooled the remaining liquor, producing a crystalline fallout.
[Edited on 4-3-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
Thanks for that, I edited the picture.
The needles (there are only a few grams) formed when I left the filtered cell contents for a few days for the black stuff to settle. The cell liquor
was cooled to approx. zero before I filtered out the main crop of K Chlorate. It took the needles a day or two to form in the liquor. The liquor
would actually have warmed up to about 10C or so and they still formed. Seems strange. Very slow growth at lowish temperature gives needles I
presume. There is always a time element to getting solute out of solvent. You must wait a certain amount of time at a certain temperature if you want
absolutely ALL the solute to come out at that temperature (I think!). Dynamics versus the figures you see in tables/graphs. The figures you see in
tables/graphs are gotton by letting a solution stabalize for quite long periods of time with the temperature fixed.
About the pH probe and interference from the cell currents/voltages.
I put my hand held, battery powered, pH probe into the Na cell (see picture of cell electodes up the thread) today with the current running. With the
probe at an inch and a half from the back of the flat Cathodes (remember the flat cathodes have a covering of plastic on the back) the pH probe read
the correct pH value. When the probe was put closer to the Cathodes errors began to creep in. Closeer to the bare wire Cathodes the probre was reading
approx. one digit too low (6 instead of 7). As the probe was moved closer to Anode the error went to 2 digits.
Your probe shoud be OK if it has a shield around it and is kept 2 inches or so from the Cathode back (You could put a covering on back of Cathode too
I guess).
A metal shield around the probe would be best IMO. Sounds like a job for Ti or Graphite, indeed.
Needing to take a hammer to a crop of KClO<small>4</small> is definitely a problem associated with success!
Hope to get around to setting up a pH controlled cell using Gouging rods (a blast from the past for me) and see how they do. One Gouging rod should
make KG's of Chlorate if erosion rates are anyway similar to EDM stuff.
Dann2
Dann2
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
I have a big clue about how to get beautiful crystals the whole way through, much nicer to work with.
My first cell was always running very hot at the top and cold at the bottom because of short anodes, thus little/no convection.
I saw this as undesirable and built my second cell with a deep anode, and I got a warm cell all the way through instead of hot and cool. I saw this a
success until I realized I was making a rock of chlorate instead of a snow-like batch of crystals.
I find the looses crystal much more desirable to work with, so I am going to rework my first cell that had short anodes (because it leaked gas and got
corroded) and use that one. I believe temperature delta makes it precipitate in the middle of the cell.
What do you guys think? Swede, if you raised up your anode to limit convection perhaps you could get a whole batch of flowing white gold.
[Edited on 5-3-2009 by y2kbugger]
|
|
|
pyro6314
Harmless

Posts: 16
Registered: 30-11-2007
Member Is Offline
Mood: No Mood
|
|
 
Im following in dann2's footsteps with the poor mans MMO until my cheap Ebay MMO comes in the slow Canadian postal system.
Since Im not pH monitoring or controlling, I understand that all the Chlorate made will be by the 9e- process opposed to the bulk reaction. Since this
is the case, temperature wont matter too much will it? Unfortunately the only place I could run this cell without (too much) complaint of the Chlorine
smell was the back step where the cell has settled at about 0*C.
Since Im not monitoring/recording/calculating anything, I have no clue when it will be close to complete. Any suggestions on when to pull the plug on
a ~2.75L cell of saturated NaCl running at ~0.8A and 3.8-4.5V (It appears to be climbing at the limited current) It has been running for approx
27hours.
@ Swede, I hope you have Cold Junction Compensation for your K Thermocouple you are trying to read. And as to our short conversation on pH electrode
poisoning; apparently if you have a liquid filled electrode that is Ag/AgCl, you can safely use it with KCl04 if you substitute the solution in the
electrode for NaCl or LiCl opposed to KCl. Apparently with KCl, Perchlorate will have the tendancy to precipitate in the junction clogging it
(poinsoning). Im still waiting for a reply back to my email requesting more information from the author.
Edit: Bottom left of first picture you can see the fan I removed from the supply that was seized, melted and causing a god awful 60Hz buzz that
vibrated the whole unit. Cheapo replacment in the works...
[Edited on 6-3-2009 by pyro6314]
[Edited on 6-3-2009 by pyro6314]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Pyro6314,
Is there a plug on the back of that thing so that you could plug it in a warm it up!!!!!!!!
I like the handle. All the cells I have ever seen they have never had a handle. I guess it has just become a 'must have'.
That cell is going to take a loooooooooong time to finish. The current is very low.
You have approx 15.5 moles Chloride total in cell (900 grams). You will convert approx. 10.5 moles of this to Chlorate. That will take 6 * 10.5 moles
electrons which is 6 * 26.8 * 10.5 = 1688 amper hours (100% CE). You have 0.8 ampes going into cell. It will take 1688/0.8 = 2110 hours to finish
cell at 100% CE. Thats 88 days. At 50% CE is will take alot more , 176 days to be precise.
Look on the bright side. At least it will be summer time when it finishes.
Put in more amps.
My understanding is that the temperature matters little when you are going the '9 electrons' route (CE 66.66%).
I once knew a guy who did a similar thing out the back in a freezing shed. He got approx. 50% CE in a cold electrolyte (call it the 12 electron
route). His gouging rods lasted well too.
BTW:
The table above in my post of 25 Feb showing CE for the pH controlled Na cell is totally wrong. I was having a bad day (another one!) when I
calculated that table. I have not got a clue how I managed to come up with the figures in it. I think I forgot to multiply by the amount of liters
(2.2) that were in the cell. Will look into it.
The overall CE was 77.7%. Total Chlorate produced was 1278 grams, total amper hours into cell was 2484.
This is similarish to the CE of the next cell I ran (KCl with pH controll, 66% CE) .
The other figures are correct. 
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Pyro6314, that power supply MUST be capable of more than 800 mA! May I ask why you are running it so gently? Is it to try and keep erosion down on
the anodes? On that coffee-pot cell, just looking at the size of both the liquor and the electrodes, I'd guess 5 to 10A would be more appropriate.
I know you are working with sodium salts. Dann2 explained the efficiency thing fairly well. For potassium salts, some time ago, I simplified the
equations as much as it was possible to do, and came up with this:
***********************************
For Potassium Chlorate Production:
W = Weight of yield, in grams
E = Efficiency
AH = Ampere-Hours used
E = (131.32 * W) / AH
For Potassium Perchlorate Production
E = (38.42 * W) / AH
*************************************
Note that these are "retroactive" equations, meaning you weigh your product, and if you've tracked the AH, you can determine your efficiency. For
predictive, IIRC it requires 161 AH per mole Chloride --> Chlorate at 100%. Note that's mole of CHLORIDE ion, not the entire salt, and if you
assume 50 to 60% efficiency, you can come pretty closely as to when it is time to yank the plug.
CJC: That little chip has it onboard! It's a wonderful device, essentially a thermocouple meter on a chip. All you need is a good power source and
a few supporting components. But when the smoke clears, I doubt I'll be using it, as I'm headed towards a suite of isolated signal conditioning
modules, gathered slowly off eBay and other sources. One of them is a PT100 RTD unit that will be more accurate for this sort of thing:

pH: I would LOVE a true pH controller setup, but the calculated dosing technique works very well. It's pretty cool when we have data like "A
chlorate manufacturer consumes X pounds of HCl per ton of product" and when scaled down, it does in fact keep the pH very near neutral or slightly
acidic. Given the problems of continuous immersion, I doubt I will do anything more than an occasional sample dip with an immediate rinse to keep the
precious pH probe healthy. Once the cell is producing, and the acid is periodically added, it seems remarkably stable; I don't remember anything
worse than +/- 0.6 swings once it settled down. It seems that the initial startup requires more acid per unit of time than later on, when the bulk
process is hopefully taking place. But with no acid - I remember seeing 9 or 10 pH within a few hours of startup.
|
|
|
pyro6314
Harmless

Posts: 16
Registered: 30-11-2007
Member Is Offline
Mood: No Mood
|
|
My graphite electrodes are hopelessly small. Only about 17.1cm2 of anode area in the solution so around 0.7A I figured was ideal to keep about
41mA/cm2 keeping anode erosion at a minimum. Plus at the cells resistance, the current draw topped out at about 1A with 4.3 ish Volts. I'm going away
for the weekend so I am going to crank it up to about 5A and leave it. (Probably risky but its outside) I wrapped some old clothing around the pot
hoping to insulate it and retain some heat but there is barely any heat running at the small current and the reasonably wide electrode spacing.
Current control is terribly sensitive with a approx 270deg pot divided for 90A. Just touch it for a couple amps. Programming ports on the back will
facilitate more accurate control with a resistor bank and pot. Only 100 Ohms full range to control current.
Im looking for a dosing pump/pH controller at present. A Hanna model will do nicely and the combo unit has a provision for manual pump control if you
dont use the pH electrode and auto control.
I dont care about my electrodes so Im going to push them hard. If they disintigrate while I'm gone, so be it. 
Edit: With two Graphite electrodes, what would an AC current do?
[Edited on 7-3-2009 by pyro6314]
[Edited on 7-3-2009 by pyro6314]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
An AC afaik does not help. It has been used (making Calcium Chlorate) to keep insoluble hydroxide from building up on one electrode but the 'AC' was
more like DC that was reversed every 10 minutes or so.
You would be better off using both rods as anodes and getting yourself some steel wire (or stainless steel) for Cathodes and increasing current.
Put a resistor (piece of Nicrome wire) going to cell to give yourself smoother current control with pot.
Regarding the CE that I reported for my first pH controlled Na Chlorate cell above ran at 20°C. When calculating CE I forgot to take into
consideration of the cell volume. I used Chlorate increase of grams per liter instead of grams increase in the cell.
All CE figures need to be multiplyed by 2.2 (2.2 liters in cell). This now gives very high CE's, up to 90%CE in the middle of the run.
I have just pulled the plug on my latest pH controlled cell ran at approx. 50°C. Graphite erosion looks similar to cell ran at 20°C.
Looks like low Chloride concentration and high pH are the real Anode murderers. (I was blaming the Butler before this!).
About pH probes in Chlorate cell. The Chlorate manufacturers may not keep their probe immerced 27/7. They may dip them in and out and wash. This would
be easy enough to for them to do. It may even be done by an operator. They probably have a known continous flow of acid going into cells (like we do)
and if pH goes to drift they stop adding or add extra acid. They would have very detailed knowledge of the acid needed for the cells as they are
operated in more or less the same region all of the time. No large Chloride/Chlorate concentration variation for years (literally) on end.
We have a harder task pH controlling our cells because of the way we operate them, (going from 330g/l Chloride / zero Chlorate at start to 80g/l
Chloride / ~400g/l Chlorate at the end). The pro's have it easy.
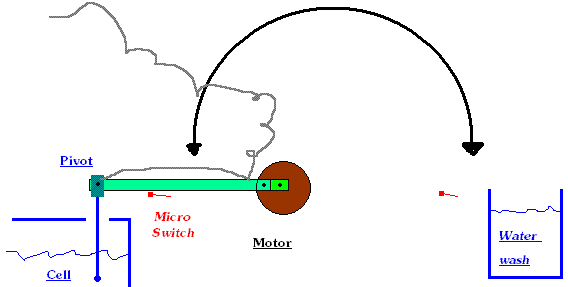
The picture below may be workable. It is more work though. You would need a permanent hole in the Chlorate tank which will not be nice if the
electrodes are in the same tank, as the horrible spray will drift out. If a two tank cell is constructed then there would be no spray from the (warm)
'chemical Chlorate formation' tank. If the motor was very weak (small stepper motor) you could forget about the micro switch beside the Chlorate tank
as the motor is not going to burn out during the fairly short time that a reading is being taken. Motor output would have to turn fairly slow too.
You are then going to have to time your taking of a reading with the probe in the tank and decide how much extra acid to add for each 00.X amount that
the pH is above optimum or how long to turn off acid if below optimum.
All easier said than done!!!
Dann2
[Edited on 8-3-2009 by dann2]
|
|
|
pyro6314
Harmless

Posts: 16
Registered: 30-11-2007
Member Is Offline
Mood: No Mood
|
|
Left my coffee pot 2.75L cell limited at 6V and it will only pull 2.2 A later dropping to 2.0A today. The anode has decreased slightly in diameter. I
have salt build up on the top of the electrodes but not much volume drop. Whoever said hotglue wont stand up to the vapors of a Chlorate cell, I have
proved you wrong. The glue is still hard and intact. The old sweater used for insulation/snow protection went from black to having a big whitish spot.
Since I have no idea of how many electrons have gone into the cell and my guess on how much NaCl was in there is most likely way off, I'm going to run
it until my graphite is gone then process the electrolyte. Ignore the PMMO cell...Real (cheap Ebay) MMO is here! Got my new fan the the DC Supply too
so that can go back together.
 
The guy shipped it to Canada for $10US since he cut the length in half. Only one small spot where the coating is wore off (pictured). Anyone have an
idea of how to accurately measure/guess the SA of this mesh?
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Pyro, your hot glue may hold together at 2 amps, but at 50 or 60 amps, it heats, softens, leaks, and becomes vulnerable to attack by the chlorine and
other gasses which pour off the electrodes at those currents. It will also deteriorate over time. That's one of the challenges of this process...
what works for some may not work for others, and finding what is consistent between users and runs is the challenge.
You'll like the MMO. It works, there's no doubt, and you'll get a clean product from it. Welding can be a challenge, but there are other ways to
attach straps, or you can cut it in such a manner that the material itself forms its own hanger. A good rule of thumb with this style of mesh is SA =
2X standard dimensions. Good luck, enjoy material. I wonder how much of that stuff he has left? I know I bought quite a bit for a rainy day.
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
I am not sure but i think that my MMO anode give my perchlorate...
before to make my chlorate cell i read that it is not possible to make perchlorate whit MMO anode but today i read a web page where they were showing
the crystal form of potassium chlorate and potassium perchlorate.(http://www.wfvisser.dds.nl/EN/analysis_EN.html) the probleme that at least 75% of the crystal of my cell have the rhombic form, wicht is suppose
to be perchlorate...
The problem is that i don't realy want tu have perchlorate for my cell because i am limited whit the use. (no HE mix and not snap bag).
Now that i am pready sure that my anode make kclo4, i have some question:
1. How can i limit the prechlorate formation. (for now i have 2 cell running in serie with a 15V 3A power supplier, they run a 20 C, no ph control,
MMO anode (from ebay), stainless stell cathode)
2. how can i be pready sure that it is kclo4 (some test if possible)
I have over 2 kg of this stuff but it is dry and in powder so i need to wait for the next batch to see the crystal(saturday night). i am going to take
pic of the crystal and show you the form of the crystal.
[Edite le 11-3-2009 par Bikemaster]
[Edite le 11-3-2009 par Bikemaster]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Going by the shape of the crystals is inconclusive at best, unless you know the purity of the solution and what all is in it. You've got a witch's
brew cocktail of various forms of KCl (presumably, since it's crystallizing) KClO, KClO3 and POSSIBLY KClO4.
As to your questions, I think you can find the answer to the second question on dann2's site: http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/...
I don't see any need to answer the first question until you know for a fact you're making unreasonable percentages of perchlorate - which you're
undoubtedly NOT doing. While the MMO anodes (various) on ebay MAY make perchlorate if you drive them really hard, I doubt you're doing that with a
mere 3A, although the 7.5v/cell is rather a lot. What's your anode/cathode spacing?
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
The space between the electrode is 5 cm.
i find i way to find the the ratio chlorate /perchlorate but i need a better scale (+- 0,1).
but i am pready sure that i have kclo4, because before to have mmo anode i was working with graphite anode and the product reacte not the same. (the
one with the graphite anode were much more unstable and reacte more slowly with atomized alluminium powder).
with chance i have and other anode... can i put an other cell to reduce the voltage??? it will be 5v wicth is pready good.
thank for your reply
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Yeah if you have more MMO mesh put another cell in series, and adjust the spacing so you get good current draw through all the cells. It's not the
best way to run things, but it will work and it won't cost you any more power than before.
If you want a perchlorate test, go to your local aquarium shop and buy some methylene blue. Make a weak solution of your crystallized product. Like a
couple crystals in 10ml in a test tube, or so. Then add a drop of the methylene blue, if you get a blue/purple precipitate, there's perchlorate.
Perchlorate contamination in chlorate shouldn't make it less stable - apparently chlorIDE contamination can, though, at least for pyrotechnic uses.
For HE, I have no idea.
Are you running the cells far beyond a reasonable completion time? That could cause perchlorate formation, but I think you'd notice some wear on the
MMO material.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tentacles
Yeah if you have more MMO mesh put another cell in series, and adjust the spacing so you get good current draw through all the cells. It's not the
best way to run things, but it will work and it won't cost you any more power than before. |
Excuse me. How is
it not "any more power than before"?
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Because 15v * 3A = 45w regardless of whether you're wasting 5v of it in resistance through the electrolyte, or if you reduce the spacing and add a
third cell to utilize that 5v for electrochemistry. The cells will run a bit cooler, but he's not making chlorate in solution anyways. Whether he can
get the full 3A across all three cells remains to be seen, but if he gets the spacing pretty close it should work out.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
If MMO could make perchlorate in any reasonable concentration, that is what the big plants would be using rather than the expensive platinum foil
sheets, with its necessary Pt recovery methods. I have tried MMO to make perchlorate, starting with recrystallized potassium chlorate, more than once
now, and it simply does not do it with any real efficiency. The best I was ever able to get was trace, no potassium perchlorate falling out, and the
solubility of potassium perchlorate is terrible - if it had been produced in quantity, it would have precipitated.
If you are starting with KCl, the bulk of the product will fall out as crystallized chlorate, and no longer be subject to much further oxidation.
Don't worry too much about the crystal shape; I've seen more than one chlorate crystal type, and it is not a reliable method to determine what you
have. If you harvest the crystals and give them a good washing during harvest, you should be in the realm of 98%+ chlorate. Recrystallize, and it
should be 99%+
|
|
|
| Pages:
1
..
21
22
23
24
25
..
48 |