| Pages:
1
..
21
22
23
24
25
..
33 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Acetone recrystallization may work or mixed solvent recrystallization may work, but generally it is difficult to get the crystals in a desired form,
and the simplest approach is to have the desired form precipitate directly from the diazotization. Then there are no extra steps, and sometimes the
product gotten directly from the synthesis isn't possible to duplicate another way.
Yes this thread has become like a clearing house for all the accumulated references available on DDNP and closely related compounds of interest. That
is probably true for some other obscure topics also. There was a huge volume of scientific research done during the WWII years that has not been
scanned and resides in vaults as hard copy and it is very possible there is some duplication to be found there if and when all of that massive amount
of data is ever published. There was a period of years there where everybody with a test tube and a bunsen burner was working on something and all
that data is archived by the government, probably a similar story around the world.
I think the acid concentration and volume is going to have great bearing at a certain temperature where the condition for the diazotization mixture
will be such that the dissolved isopicramic acid salt will be nearing but not quite a saturated solution at that dilution strength of acid, and
byproduct H2O from the diazotization will not be sufficient to cause precipitation of the undiazotized soluble isopicramic acid, but will very
gradually disrupt the solubility for the soluble diazonium nitrate by the presence of the byproduct H2O so that slowly crystal growth occurs for the
precipitating p-DDNP. So you have those factors in play for what would determine the optimum acid concentration, provided in sufficient volume to
keep the undiazotized isopicramic acid dissolved, but allow for hydrolysis of the soluble diazonium nitrate to the p-DDNP that would be not fully
soluble in that HNO3 dilution, which is becoming more dilute as the reaction proceeds. Some of the HNO3 will be consumed by reaction with the copper,
which has the effect of dilution added to the byproduct H2O from hydrolysis of the diazonium nitrate which increases the dilution......so the reaction
system is trending toward greater dilution during the entire course of diazotization. That means there needs to be sufficient excess of acid at the
beginning for the reactions to run to completion. If only a little further product precipitates when the finished mixture is diluted with additional
water, then the "try proportions" which were used are likely very close to optimized with regards to crystal growth. Sometimes a little of the total
possible yield is lost as the cost for a slowed reaction growing the best crystals, versus a rapid reaction which produces higher yield in terms of
total weight, but may produce a less pure and more amorphous product.
[Edited on 9/17/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Very right...though may I add that perfect 0.5 cm long needle shaped crystals are best avoided?  Crystal breakage, static, low density etc. Believe me, I've seen them for p-DDNP. It happens for very dilute solutions
of the isopicramic chloride/sulfate at very low temperatures. When recrystallized from boiling dH2O/1% HCl, pDDNP formed almost hair like crystals,
very thin,long and flexible, almost like wool. Recrystallization from acetone is not necessary, when boiled too long, it can even decompose part of
the product and lead to less purity. On the other hand, residual sulfuric acid in the product from copper/SA is adds an unknown to long term
stability. It's interesting though, one of the culprits of DDNP has been obtaining a product with high bulk density. Both times the copper method gave
a bulk density that was incredibly high, first time I thought my yield was terribly low and contemplated to throw the batch away without weighing,
glad I did. Crystal breakage, static, low density etc. Believe me, I've seen them for p-DDNP. It happens for very dilute solutions
of the isopicramic chloride/sulfate at very low temperatures. When recrystallized from boiling dH2O/1% HCl, pDDNP formed almost hair like crystals,
very thin,long and flexible, almost like wool. Recrystallization from acetone is not necessary, when boiled too long, it can even decompose part of
the product and lead to less purity. On the other hand, residual sulfuric acid in the product from copper/SA is adds an unknown to long term
stability. It's interesting though, one of the culprits of DDNP has been obtaining a product with high bulk density. Both times the copper method gave
a bulk density that was incredibly high, first time I thought my yield was terribly low and contemplated to throw the batch away without weighing,
glad I did.  Tomorrow I'll see if I can reproduce it and trow in some 40x
manification pictures. Tomorrow I'll see if I can reproduce it and trow in some 40x
manification pictures.
The p-DDNP from the isopicramic obtained directly after deacetylation in general yields glittering p-DDNP with a silver/golden sheen, which only
happens for very pure DDNP in general.
[Edited on 17-9-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I wonder what using the copper in a mixture of nitric and acetic acid might produce. Any residual acid trace would be volatile, and p-DDNP is stable
to acidity anyway, so trace acid would be likely not to adversely affect storage. If the base charge was likewise something that had no issue with
acidity, like guanidine perchlorate or methylamine perchlorate, or trimethylamine perchlorate, the combination could have long storage stability and
would be very economical.
[Edited on 9/17/2015 by Rosco Bodine]
|
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
So, a sufficient concentration and volume of Nitric acid is needed to keep the isopicramic acid dissolved in solution but at the same time allow for
hydrolysis of the Diazonium nitrate salt.
There also needs to be a sufficient amount of Nitric acid so that the reaction can run to full completion. Hmmm.
My iso-picramic acid is not fully dry so I have placed it in the desiccator on paper towel using Calcium chloride as the desiccant in a dark cupboard
to speed things up.
It looks like after i have used the nitrate salt/copper method I may have to try the diazotization with 15, 17.5 and 20ml HNO3 in seperate runs but
also varying the cocentration between 20-35%. Any idea what combination would be a good start?
[Edited on 18-9-2015 by greenlight]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Trying to offer an off the top of my head, what I think could work kind of guess, without doing the stoichiometry first and having any experimental
observations as a guide, this is purely a shot in the dark kind of guess about the range of HNO3 concentration that may be useful. I'm purely
guessing the HNO3 concentration range would be about a median 35% plus or minus 10% depending on the volume ratio in relation to the isopicramic acid,
higher volume of HNO3 per gram of isopicramic acid allowing for the lower concentration range. Total HNO3 decomposed in reaction with the copper
along with the starting and byproduct H2O should result in an endpoint HNO3 concentration having dropped to about 20% from the more concentrated level
it began. This is totally an off the top of my head guess. This is really more an aspect of the diazotization reaction about which nitro-genes can
better provide guidance.
A variation on the process that could make use of the lower concentration of HNO3 would be to start a drip of concentrated HNO3 being gradually added
to maintain the acidity relatively constant over the course of the diazotization, adding dropwise the needed "makeup quantity" HNO3 to replace the
HNO3 being consumed by reaction with the copper, maintaining the HNO3 concentration in the reaction mixture around 20% or whatever very near to that
concentration is found to keep the copper reacting actively.
You could do the math and formulate the reaction condition I described as a kind of starting point for "try proportions" and see how it works. There
exists the possibility also that the sulfuric acid content is needed or changes things in a way that is required to get the high density product
reported by nitro-genes. It might not be possible to take out the H2SO4 from the reaction system without upsetting the process, so it really is
unknown whether a modified scheme absent the H2SO4 will work as well.
Experiments are being done by nitro-genes to develop more information so this is a work in progress.
[Edited on 9/18/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
In reponse to a U2U from Greenlight, and after rerunning the nitrate/SA/Copper diazotization, the method outlined in the prepublication section
doesn't work, I couldn't reproduce the results I posted earier. It seems the reaction needs a minimized water content to proceed using only mild
heating. Most likely, I left the reaction on the heater plate for longer period of time, for which the water content may have become lower than that
what was started with. I've redid the reaction and tried to replicate the results, this time monitoring the reaction.
To produce the very dense crystals is more tricky than I thought, if there is a large exotherm from the reaction with copper, there is no time for
crystal growth and almost amorphous DDNP seems to be formed.
Best result (with minimized quantities of reagents) was obtained by the following method:
- While stirring, 3 ml of 96% sulfuric acid was added to 3 ml water in a 25 ml beaker and left to cool to below 50 deg C.
- Than add: 2.2 g KNO3, 1 g isopicramic acid, and 2 gram of copper ("beads" from electricity wire)
- Give it a good swirl, to break up any remaining lumps and put some clear foil over the beaker to prevent NOx from escaping
- Keep the mixture between 40 and 50 deg C. for one hour while stirring, wash with water several times, filter and wash until filtrate is not green
anymore
Very dense, light orange-brown p-DDNP, yield will be determined (its not dry yet) and I'll add some 40x magnification pictures of the resulting
crystals in the coming days too. 
Very interesting how the reaction does not proceed at all when water content is above a certain threshold, although NO is produced (fizzing of
solution) and the isopicramic just floats around, it isn't oxidized or something. Maybe the NO2 is indeed doing the work here...puzzled...
[Edited on 24-9-2015 by nitro-genes]
|
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Thanks Nitro-genes, I will run it that way and see how it goes.
Did you crash the final solution after heating into ice-cold water or water and crushed ice in it too?
Could larger crystal sizes be achieved if you add the final solution after heating to an addition funnel and drip it into ice water with stirring?
[Edited on 24-9-2015 by greenlight]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@ nitro-genes When you first described the use of copper for the diazotization it was done first forming the soluble acid salt of the isopicramic
acid, and then diazotizing that solution of already dissolved isopicramic acid sulfate, like is the dissolved and filtered product from the
deacetylation mixture. Your first post about this was here.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
Anyway I had the understanding it was the diazotization of the already in solution isopicramic acid that would produce the best crystals.
Maybe you just noted some figures incorrectly and didn't duplicate results because the second attempt is using conditions that are actually not
exactly the same in some detail.
I know from experience that some reactions are very fickle about a precise kind of narrow window for reaction conditions and very small changes that
would seem like should be of no consequence will have an unexpected drastic effect. What may seem like should be a trivial detail isn't trivial for
such reaction scenarios when you have a reaction that is sensitive and may require exacting conditions. It is understandable that a diazotization
like this with the algebra involving solubilities being a factor as it definitely is, would likely be sensitive to the acid concentration and
temperature and sequence of addition.
There is probably a pretty narrow range of exacting conditions required for the crystallization to be optimized.
One of the factors involved here is I think the amount of dilution of the acid concentration that can be tolerated at a certain temperature, for the
soluble isopicramic acid sulfate (or nitrate or sulfate / nitrate mixture) to remain in solution. Earlier tests were done to identify generally what
is the dilution at which the solubility is disrupted and this is important to know. The acid concentration should be kept high enough so that the
solubility of the isopicramic acid is assured, and the concentration of the acid is also high enough for the reaction of the copper. If the reaction
proceeds as I have supposed, then the product of diazotization is expected to have less solubility in that acid concentration for the reaction
mixture, and should precipitate gradually. There is an unknown "sweet spot" for the acid concentration at which the diazotization reaction will
proceed to produce the best crystals. Ideally there will be a specific acid concentration and temperature combination that is optimum for the
process. But there is a dynamic involving the changing acid concentration due to the consumption of the HNO3 in the reaction with the copper, which
has the effect of an ongoing greater dilution of the acid. With larger volumes of acid used for the reaction mixture the change would be lessened to
a smaller range. Or in the alternative, the acid concentration could be maintained at some concentration identified to be optimum by a gradual
addition of HNO3, to replace the HNO3 being consumed in reaction with the copper.
The same effect of adding "makeup" HNO3 should occur by adding in increments a nitrate salt to the H2SO4 reaction mixture, so adding the nitrate in
small portions over time could be helpful in refining the process. Keeping the temperature and acid concentration constant during the diazotization
would be good to make those variables minimized and would simplify identifying what is the optimum reaction condition with regards to time of reaction
and acid concentration and relative quantity / volume ratios for the reaction mixture.
Process algebra is the fun required to optimize a synthesis.
[Edited on 9/24/2015 by Rosco Bodine]
|
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I just attempted Nitro-genes revised last step from isopicramic acid to DDNP.
1 gram isopicramic, 2 grams copper beads, 2.2 grams Potassium nitrate, 3 ml 96% Sulphuric acid and 3 ml distilled water were used.
Heated for an hour at about 46-47 Degrees celcius with clear foil wrapped around the beaker to prevent escape of NOX before being poured into ice cold
water and filtered.
Does this look right before I get my hopes up waiting for it to dry?
[Edited on 25-9-2015 by greenlight]
   
|
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Disregard the above results, the product is not DDNP at all, the colour looks wrong too.
I had another attempt today with the same procedure and even added about 1 ml of 30% HNO3 half way through to see if that improved things, but still
no luck . .
All my reagents are pure and the copper beads are the right size, the only things is I don't get a greenish tint to the brown solution that
nitro-genes speaks of at the end of the hour heating at 50 C.
Maybe I am not heating for long enough.
[Edited on 26-9-2015 by greenlight]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Did you try what I suggested about the order of addition, dissolving the isopicramic acid in the acid first to form the soluble acid salt? I would
have to go back and review all the old references and I don't have time, but IIRC the soluble acid salt of isompicramic acid once formed would
withstand dilution and should remain in solution in still relatively acidic low pH solution. I think nitro-genes confirmed this with regards to the
dilution of the deacetylation mixture not causing precipitation of the soluble isopiramic sulfate, which remained dissolved, while impurities were
precipitated and could be separated by filtration.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
The soluble isopicramic acid sulfate is reactive to diazotization and the diazotized product is more inclined to precipitate depending on the
dilution, than the unreacted precursor. So I think the order of addition could have bearing.
Translating what I am trying to say, for example where nitro-genes diazotizes a suspension of isopicramic acid in 10% HCl, it may be better to first
dissolve the isopicramic acid in 1 volume of 30% HCl and dilute that solution with 2 volumes of H2O to obtain the same reaction system of isopicramic
acid in 10% HCl but in solution rather than in suspension, and then diazotize the solution.
That's the way I would try it anyway, is work with solutions of reactants because the contact is more intimate for the reactants already dissolved.
The difficulty for a solid precursor in suspension is having to react by diffusion through a surface reaction coating and erosion of solid particles
underneath. The subsurface of the particles are tending to be passivated by a nearly insoluble overlay coating of p-DDNP that is a surface reaction
layer that is sealing off the unreacted sold beneath. Ideally, I think what is desirable is for all the undiazotized isopicramic acid sulfate to
remain in solution over the course of the diazotization.
[Edited on 9/26/2015 by Rosco Bodine]
|
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Okay thanks Roscoe, I will try that method next.
I will try to dissolve all of the isopicramic acid in the sulfuric acid first using Nitro-genes amounts again and then dilute with distilled water
before adding the copper beads and Potassium nitrate.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I was actually curious about some of these process details in reviewing the pre-publication writeup, because for the example [2] there is the method
described of putting the reactant isopicramic acid into solution first. From a process standpoint that is desirable. Likewise from a process
standpoint the nitrate that would seem best of the 3 would be sodium nitrate. Solubility is better for the byproduct sodium acid sulfate than for the
potassium salt, and for the ammonium nitrate even though the solubility is best, the ammonium salt would work to decompose part of the nitrous acid
being formed so it would be less efficient and probably not work as well as either of the non-ammonium salts.
There are several possible methods for diazotization that are different from any of the 4 methods which nitro-genes has described. A possibly useful
method would be to form a solution of the potassium salt of isopicramic acid and mix with a solution of potassium nitrite and very very slowly
dropwise add to the stirred solutions dilute HCl or possibly HNO3 or acetic acid, taking perhaps 2 hours to complete the addition and keeping the
temperature in a range of perhaps 25-35C. A similar method was found to produce a high density product for o-DDNP and may work also for p-DDNP with
the right combination of reactants and conditions. Sometimes a very specific combination of particulars which define a narrow window for a reaction
will produce excellent results.
It might likewise be interesting to try potassium isopicramate solution in water or neat isopicramic acid in a mixture of water and alcohol and to
diazotize by bubbling into the solution through a gas diffuser fine bubbles of nitrous gases produced from HNO3 plus starch. This could provide
economy compared with the use of copper and eliminate copper recovery for recycling in large scale work.
http://www.sciencemadness.org/talk/viewthread.php?tid=3033&a...
Likely the byproduct of a diazotization of a potassium isopicramate solution using nitrous gases would be a solution of potassium nitrite, which could
be recovered and used in an alternate diazotization method if desired. Possibly the diazotization could be conducted only to the midpoint of
completion using the nitrous gases, and terminated, with the diazotization completed by a gradual addition of an acid to react with the potassium
nitrite already produced, which should diazotize the remaining unreacted potassium isopicramate. This method is possible on paper as theory and is
not certain would work as anticipated, but likely would work.
A concurrent very gradual dropwise or metered injection addition of an acid could be made along with the nitrous gases, lagging somewhat behind the
gassing reaction rate, to shorten the reaction time and have the parallel diazotization reaction schemes operate simultaneously.
Neat isopicramic acid dissolved in alcohol likewise should be diazotized by nitrous gases, which I think is the original method of Griess which was
used to produce o-DDNP and should likewise work for p-DDNP.
[Edited on 9/28/2015 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
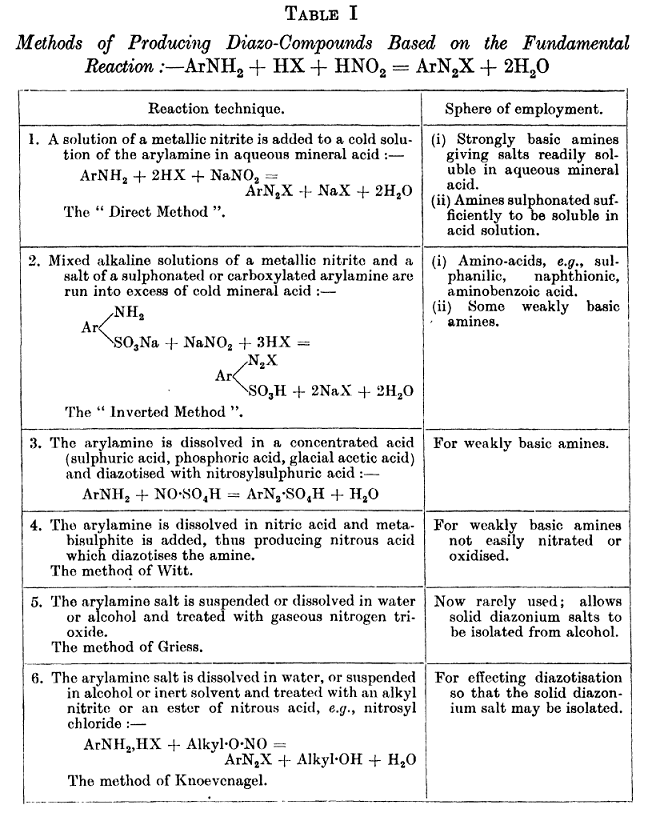
textbook download links
Here are a couple of textbook download links that are for books provding some general information
The Aromatic Diazo-compounds And Their Technical Applications
by Saunders, K. H Published 1936
https://archive.org/download/aromaticdiazocom031270mbp/aroma...
The Chemistry of the Diazo-compounds
John Cannell Cain, Published 1908
https://books.google.com/books/download/The_Chemistry_of_the...
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
@Greenlight, did you see any red coloured gas (NO2) above the reaction mixture? The copper should be oxydised, releasing NOx which should result in a
clearly visible red gas in the reaction vessel and results in a greenish solution, since that is the colour of the Cu2+ x HNO3 ions formed. It is
also important also that the reaction doesn't run out of control and gets much hotter from the exothermic oxidization of the copper, I think I got the
brown crud also when the reaction runs too hot by itself at the start. The last reaction were monitored constantly and heat was only applied after
mixing everything, just until generation of NOx became evident.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
crystallization modifiers
There was earlier in this discussion a speculation that there may be a modified crystal formation possible through a cocrystallzing of the two
different isomers of o-DDNP and p-DDNP and I have found an obscure reference which tends to support the idea of the one isomer operating to modify the
crystallization of the other, described for the case where o-DDNP is crystallization modified by the presence of p-DDNP in small amounts. I believe
it is possible the reverse scenario is also true, that the presence of the one isomer of DDNP will work to modify the crystallization of the other.
See patent US2103926 which describes this effect. Also are listed a series of other materials which can also accomplish a modifying of the
crystallization.
See the screenshot from that patent, middle of line 8 "isopicramic acid" is shown as a crystallization modifier for ordinary o-DDNP, but under the
reaction conditions it is a given that it is probable the effect is also from p-DDNP and the distinction is unclear.

Attachment: US2103926 DDNP manufacture.pdf (382kB)
This file has been downloaded 714 times
|
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
@Nitro-genes, no, not much visible red NOX gas was seen at all. The final solution did not have a greenish colour either it goes dark red halfway
through and red-brown at the end. Temperature never goes over 50 Degrees either.
I don't think that I am heating for long enough that's why I am not getting the greenish colour to the end solution. I will try again and dissolve
the isopicramic acid in the acid before I add the nitrate salt, water and copper like Roscoe said.
I will heat at a lower temperature like 40 Degrees and continue heating until the greenish end colour is obtained.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
OK, here as promised the photos of the nitrate/SA/copper diazotization:
Left column are from nitrate/SA/copper diazotization described earlier, right column is also very dense and free flowing p-DDNP, produced by slowly
adding a sodium nitrite solution (30 minutes) to a isopicramic acid/SA solution at 30 deg C. Short story, slow nitrite addition makes best rounded
crystals, though the nitrate/SA/copper method produces slightly cleaner pDDNP, as was to be expected, but more agglomerates of tiny crystals (hard to
see in photo).
@Rosco, I'll try the o-DDNP/p-DDNP diazotization, any suggestions for best approach? 

[Edited on 30-9-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
What would work best for a crystal modifier scheme is total guesswork so it is a shot in the dark to just try things and develop and refine what
works. As a starting point I would try 1% of the other isomer precursor in the same reaction condition introduced as a deliberate impurity. It could
require a lot less, but just a guess is that something in the range of 0.1% to 1% should do the job.
The use of the potassium salts for the picramate and or isopicramate and potassium nitrite with the use of nitric acid for the diazotization acid has
always seemed to be a logical combination since any impurity byproduct from the diazotization would be potassium nitrate which is an oxidizer and is
not hygroscopic so it would not tend to be an impurity in the product that would cause any issue. A neutral and not too concentrated solution of
potassium isopicramate, with perhaps 1% potassium picramate as a modifier, and potassium nitrite at something over the amount required by theory
could be diazotized by dropwise addition of dilute nitric acid., or better by a very slow infusion of the dilute HNO3 through a fine capillary or
through a dispersion frit into the stirred mixture at maybe 20C. A liquid flow through a dispersion frit can be very slow addition like a one drop per
minute gravity flow where the liquid slowly "weeps" through the porous membrane as a film washed away into a stirred liquid. The effect is much the
same as using an atomizer for the acid being fogged into a carrier air stream, it accomplishes a very fine microdispersal of the acid and gradualizes
the reaction interface which promotes crystal growth. The technique is described in US2155579 attached, but the same result can be approximated by
using a frit as described. Combining the benefits of both the crystal modifier schemes that involves both the reactants and the physical manner of
addition could even more enhance the quality of the crystals which result.
Some of the other modifiers that are listed would be worth a look also and the methyl orange in particular struck me as interesting because it is a
dye and there is another patent that also teaches that certain dyes can act as crystal modifiers for o-DDNP and would likely act the same way for
p-DDNP. See US2408059 for the use of specific dyes that have been found to modify the crystallization of o-DDNP which may or may not work also for
modifying the crystallization of p-DDNP.
Just a note about the stoichiometry that applies to the copper method: If the reaction of copper was completely efficient and all of the produced NO2
reacted to accomplish the diazotization, each 1 gram of copper would diazotize 6.26 g isopicramic acid as 100% of theory. How efficient is the actual
real world reaction would depend on several factors, including the shape of the reaction vessel. Something tall and narrow like a cylinder shape
would allow more of the rising bubbles of NO2 to be absorbed and reacted with the liquid, before escaping at the surface and being lost from the
reaction. Reactions at normal pressure where the reactants are in different phases like a gas being absorbed by a liquid are not perfectly efficient
because the rising bubbles of gas don't get fully reacted and some unreacted gas escapes and is lost unless there is a recycling for multiple passes
where incrementally it is absorbed and reacted little by little additionally on each pass. It is the very gradual reaction that is rate limited there
that allows for crystal development. Some schemes where the gassing method is used the gas is inert to the reaction but operates as a carrier for a
liquid reactant that has been fogged into the gas stream using an atomizer of some kind. That avoids the local reaction zone that occurs when an acid
is added by drops like would fall from an addition funnel, by microdispersal of much smaller drops that results in a more homogenous mixing and a
gentler shifting of the reaction zone so there are no drastic differentials from a less precise mixing. The effect of reaction at the interfaces is
slowed down for materials which react almost instantly on contact so that crystals have more time to grow.
The reason the copper method works to allow for the crystal growth is because the nascent bubbles of NO2 are so microscopically small and the
dispersion is so gradual. The effect would be amplified if a solid wire was attached to an unltrasonic transducer and the vibrating surface of the
copper would release the tiny bubbles of NO2 even more readily, and the smaller the bubbles are the better they are completely reacted before they can
rise to the surface and escape as loss.
Attachment: US2408059 crystallization modifiers.pdf (371kB)
This file has been downloaded 633 times
Attachment: US2155579 Manufacture of DDNP.pdf (255kB)
This file has been downloaded 644 times
[Edited on 10/1/2015 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
After some more experiments regarding how the synthesis conditions for p-DDNP affect crystal shape, it seems the most influential condition
determining the formation of plate like structures or almost spherical crystals seems whether the starting point is a completely clear solution of
pDDNP in a strong acid, or from undissolved isopicramic as a suspension, the former producing plates, the latter more rounded structures when the
nitrite is added slowly. I'm guessing the crystal shape of the isopicramic itself may be very important in determing crystal shape. The isopicramic
obtained after deacetylation using sulfuric acid consists of sort of spherical aggregates of tiny needle or plate like structures as described by
Dabney. Purely guess work, but perhaps by careful maintaince of undissolved and dissolved isopicramic acid these spherical like structures are both
diazotized and "filled up" by acting as nucleation points for the p-DDNP formed from the soluble isopicramic acid part, which in combination with
strong stirring may lead to the spherical shapes.
Diazotization of the potassium salt of isopicramic was tried before, not sure if I mentioned it though. Even when kept cold, the basified isopicramic
seems to produce numerous dark coloured byproducts when the acid is added slowly, I don't see a way to do this, other than perhaps a carefully choosen
slighlty acidic buffer solution. Plunging in the acid instead of adding drop by drop is no problem and gives good quality p-DDNP, so most likely the
first additions lead to minor HNO2 formation in an environment that still has a too high pH, leading to oxidation, as is evident from the fact that at
a certain point during the additions, it turns very dark. Maybe checking pH at this point and performing the reaction in a buffer slightly more acidic
could prevent this.
Some 40x photos of experimental diazotization schemes will follow. 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There is likely a specific concentration for the HNO3 solution of isopicramic acid useful for the diazotization which would provide good results and
produce no trace sulfate or sulfuric acid impurity. I don't know what you found was the solubility ease for the isopicramic acid in HNO3 and what was
the limiting HNO3 - H2O concentration / temperature relationship there. For the copper used directly any copper nitrate impurity would be
hygroscopic, so use of potassium nitrite would be indicated as preferable there, or using an external gas generator and gas dispersion into the HNO3.
If the crystal modifier used is 1% picramic acid it should dissolve in the HNO3 also. Have you tried something like this?
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Quote: Originally posted by Rosco Bodine  | I have an idea for a possible alternative method of synthesis for o-DDNP based upon nitration of salicylic acid to 3,5-dinitrosalicylic acid,
followed by reduction by sugar to 3-amino-5-nitrosalicylic acid. This would be subjected to a combined diazotization and further nitration with
accompanying decarboxylation to form o-DDNP.
Attachment: Dinitrosalicylic_acid_as_a_reagent_for_the_estimation_of_sugar.pdf (344kB)
This file has been downloaded 24 times
Attachment: Meldoa et al 5-Aminosalicylic acid and derivatives 533.pdf (326kB)
This file has been downloaded 29 times
Aspirin could be used I think as the starting material since it would be easily deacetylated by the nitration mixture leading to 3,5-dinitrosalicylic
acid. The sodium salt would be formed and dissolved in hot water for reaction with fructose or glucose.
[Edited on 9/1/2015 by Rosco Bodine] |
Recently did some experiments regarding the synthesis of 3-5 dinitrosalicylic acid (DNSal), seems like an interesting compound since it may be useful
for several purposes:
1. Determination of glucose concentrations by reaction under alkaline consitions to produce the bright red coloured 3-amino-5-nitrosalicylic acid
2. Conversion of the resulting 3-amino-5-nitrosalicylic acid to o-DDNP
3. Reaction with excess urea may produce 3,5 dinitro salicylamide, which after hofmann rearrangement may produce 3,7-dinitro benzoxazolone (Wasn't
able to find this compound, so it may not exist or cannot be synthesized this way, hypochlorite may also interact with the benzene ring since the
nitrogroups promote formation of all kinds of meissenheimer complexes under basic conditions (chloropicrin?). So not sure about this one, although
supposedly m-nitro aniline can be formed by hofmann rearangement of 3-nitrobenzamide. If this would work however the 3,7-dinitro benzoxazolone may be
hydrolysed under mild conditions by a strong acid to yield a soluble picramic acid salt that can be titrated with a base to yield free picramic acid
(analogous to isopicramic), which would be a hydrolysis and purification step in one. This route may have some advantages over reduction of picric,
since in my experience benzoxazolone is stable to further attack during the hofmann rearangement, whereas over or under reduction of picric is always
a danger, as well and canbe obtained in very high yield as well as that the reduction of PA can produce difficult to filter sulphur particles.
Further nitration of 3,7-dinitro benzoxazolone may also be interesting, though perhaps not possible without hydrolysis.
6. Salts of DNSal may form interesting pyrotechnic compositions with chlorates/perchlorates
Anyway, where was I?  Ah yes... DNSal synthesis Ah yes... DNSal synthesis
The synthesis protocol for the first article is pretty limited regarding detailed information. I attempted the synthesis using 10 grams of salicylic
acid from hydrolysis of aspirin, only replacing the large excess of nitric acid used by an equimolar amount of ammonium nitrate and sulfuric acid. The
reaction was kept between 5 and 10 deg C during the additions (45 minutes) and was stirred at 0 deg C. for an additional hour before crashing on ice.
After recrystallizaton from boiling water, there were 3 distinctly seperate colours and crystal structures present, melting point of between 120 and
140 deg C. The solution further darkened pretty fast in sunlight, which lead me to believe that the product was mostly composed of 3 and 5 mononitro
salicylic acid, together with a small amount of DNSal. Obviously, the presence of the deactivating nitro and carboxyl group prevents dinitration at
these temperatures. The synthesis utilized by Meldola uses a very large excess of fuming nitric acid to overcome this. During the synthesis of piric
acid there seems to be however very little decarboxylation going on below 60 deg C. So I tried the synthesis again heating in increments to 50 deg C
to see if DNSal may be produced at higher temperatures and without an extreme exess of fuming nitric acid. Also here the use of AN may have some
benefits over NA, since the formation of some of the NOx secies responsible for the decarboxylation (nitrous acid for example) may be hindered by the
presence of the ammonium. The procedure looked something like this:
70 grams of 97% sulfuric acid was poured into a 250 ml glass beaker. The beaker was added to an icebath together with a thermometer put on a magnetic
stirrer plate, after which 12.5 (2.2 molar eqv) grams of completely dry ammonium nitrate was added and stirred for 5 min to dissolve. When the AN/SA
solution was cooled down to 0 deg C., 10 grams of finely powdered salicylic acid was added in about 0.5 gram amounts while keeping the temperature
between 5 and 10 deg C. The exotherm is a mean one and lags considerably, so the total addition took almost 45 minutes to complete. During the
aditions the mixture takes on a very slight yellow colour. After the final addition the mixture was stirred in the icebath for another 30 minutes,
after which the mixture had thickened considerably and looked like unwhippped cream (off white colour, slight yellow tint). The beaker was then
removed from the hotplate and allowed to slowly come to room temperature while stirring and kept there for another 30 minutes. Then the mixture was
slightly heated to 40 deg C. for another hour, no decarboxylation was observed yet, viscosity was much lower and stirring easy. After briefly heating
to 50 deg C, I thought to see some gas formation and icecold water from the ice bath was added to a total volume of 250 ml. The mixture was prety thin
after adding the water and needed to be put at 4 deg C. for several hours for precipitating the product. After 1 hour the entire mixture had
solidified to one mass. After stirring the suspention briefly with a thermometer to loosen things up, it was filtered, washed with 300 ml of icecold
water and put in a clean 250 ml beaker again. To this was added 150 ml of boiling dH20 to dissolve completely and left to cool to 4 deg C in the
fridge. As Meldola observed, the light yellow solution is prone to supersaturation, and was cooled almost completely when crystallization commenced.
The crystals look much cleaner this time, sort of large spherical aggregates of cream coloured needle shaped crystals, clining together as one solid
mass. Sort of resembles a heavy crowd of sea urchins. (Hehe, there is probably a more professional word for this).
Total yield after drying was 16.17 grams of DNSal from 10 grams of salicylic acid. This would represent a 97.9% yield, which seems a bit high. Maybe
there is some TNP contamination explaining the high yield, or perhaps DNSal forms a hydrate. For the monohydrate, the yield would translate to 90.7%.

The dry material behave peculiar on the hotplate. When heated fast, it briefly partially melts at around 150-160, only 30 seconds later to solidify
again, thereafter melting at around 180 deg C. Best bet would be DNP/MNS contamination, which probably sublimes at around 150, leaving only DNS
behind. Other explanation would be it releases its hydrated water. Opinions?
 
[Edited on 29-10-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=8815
This looks interesting that the DNSA can form acid salts or at least it reportedly forms one acid salt with lead. This might possibly be a basis for
potential multiple salt complexes, if an acid DNSA salt could further react with a basic salt or possibly a basic complex cation to form a neutral
multiple salt. For example I was thinking that perhaps the Lead DNSA acid salt might react with 2 basic lead picrates to form a bridged neutral
double salt.
The extremely low solubility of precursors would require a very long digestion of highly agitated suspensions of the lead DNSA acid salt with 2
equivalents of basic lead picrate to form the contemplated neutral complex salt, that is of course IF it exists. This is purely off the top of my
head experimental theory that may or may not exist as an actual complex salt and contemplated "designer" material. If it does exist it could be
interesting.
The patent mentioned in the other thread is attached
and the Lead acid salt of DNSA has interest by itself.
US2021497 Lead Salts of Dinitrosalicylic Acid
Attachment: US2021497 Lead salts of dinitrosalicylic acid.pdf (221kB)
This file has been downloaded 604 times
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Thanks for looking up that thread, post by ozone is interesting and matches my experience with glucose reduction of DNS. When I tried the reduction of
alkaline DNS (2.28 g) with glucose (1:1.2) the solution goes bright red upon boiling initially, than a sort of brown red, then when cooled and
acidified to a pH of 3, some funky autumn coloured compounds seemed to precipitate, the majority dark brown, with some additional orange and red
stuff. I doubt it is a very specific reaction, although recrystallization attempt was not made. The reaction doesn't proceed at all when excess
ammonia is added instead of NaOH, and seems to need at least 3-4 molar equivalents of NaOH.
Might this be interesting? The article describes direct formation of diazonium compounds from salicylic acid in buffered nitrite solutions. Can't see
how this would work, perhaps via partial reduction of the nitrite to nitroso or hydroxylamine? Maybe the acetone or perhaps part of the salicylic acid
is part of the reaction mechanism? http://pubs.rsc.org/en/content/articlelanding/1959/jr/jr9590...
That would be something... DDNP directly from DNS and nitrite
[Edited on 31-10-2015 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That is interesting and it also appears to be a slow reaction so it could form good crystals. Should get the complete earlier reference part IV and
this reference part V to see how this is being done.
|
|
|
| Pages:
1
..
21
22
23
24
25
..
33 |