| Pages:
1
..
20
21
22
23
24
..
60 |
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
I think that given that paper, if the residue is non-reactive at ordinary temperatures (and neither is pure phosphine), it may even be possible to get
Red Phosphorus from a fairly simple procedure at STP.
quam temere in nosmet legem sancimus iniquam
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
That has been posted here before. Mind you, there isn't much that hasn't been posted here before!
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
sorry bout that...you have to admit it's pretty cool though. 2 flowerpots, sand, fire clay, and a couple of coal or graphite art pencils. the weekends
are getting hard to sit through. knowing my reactants are sitting on a truck somewhere and UPS doesn't move on the weekend...patience though...i can't
rush through the experiment...
i have seen in various places that you cannot gas red phosphorus to get PCl3. but i ran across this article a while back about the production of
phosphorus oxychloride from phosphorus thichloride derived from red phosphorus. am i misinformed?
"charge your rxn flask with dry RP powder. lead the Cl2/helium line through one stopper (use cork POCl3 attacks rubber stoppers) the center
one is equiped with a glass stir bar that reaches the bottom with atleast 5cm to spare, the end in the flask has had a short section of glass rod
melted to it crossways. The last neck has a hose leading out a window or to a hood if you have one. The flask is now charged with helium, not helium
for balloons, that stuff is only about 35% helium and the rest air. get a small cylinder from a welding store, nitrogen will work in a pinch but you
want a light gas. If you were to use argon it would blanket the RP and prevent the Cl2 from getting to it. Now that your flask is inerted, slowly
(REALLY SLOWWWW...) turn on the supply of Cl2. The trick here is to let the chlorine 'trickle' in in small amounts (PPH amounts). If you put too much
in the RXN goes exothermic and will produce flame, possibly an explosion if you have a large enough amount of RP or melt through your flask creating a
dangerous chemical fire. If you do it right you can feel the flask by the RP and it will be warm possibly hot but not flaming. Gradually the RP will
start to look 'wet' as the phosphorus trichloride starts to form you may have to break up and stir the RP from time to time to get it all to react.
Eventually you will have a puddle of PCl3 with some RP that did not react. Now you can turn up the chlorine a little bit and start stirring to react
the rest. Once the liquid is as clear as it is gonna get flush the flask with helium again and insert a section of glass tube into the liquid. You now
have to pass pure O2 through it for several hours.
sorry i can't provide a source. i copied this text and saved it in my notes some time ago. also sorry if this is the wrong place for this but i saw
mention of PCl3 earlier in this thread and that is a compound i am interested in but if it can't be done with red phosphorus that would be good to
know...i'm hesitant to try it with white phosphorus because of how reactive it is.
[Edited on 22-8-2010 by Rogeryermaw]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
P is amazingly reactive with most non-metals.
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
What has been posted here before? The reaction of Phosphine with Dimethylchloramine? If so, was it tried? I'd prefer to know if it doesn't work before
anyone tries it...
@Rogeryermaw
If you have a look, the preparation of POCl3 from P4O10 & NaCl (per Tarbutton, et al) in a tube very like the one I saw on the previous page (starting at about 250'C
IIRC) is also "reported" (from a VERY RELIABLE source, ie. not me  ) to work
"just as the paper says". ) to work
"just as the paper says". I'll throw up two of the papers cited by the paper in
question, as they are rather instructive. I'll throw up two of the papers cited by the paper in
question, as they are rather instructive.
[Edited on 23-8-2010 by aliced25]
Attachment: Lucas.Ewing.New.Method.of.Preparing.PF5.pdf (306kB)
This file has been downloaded 710 times
Attachment: Thorpe.Hambly.On.POF3.pdf (83kB)
This file has been downloaded 635 times
Attachment: Tarbutton.POCl3.from.P2O5.NaCl.pdf (802kB)
This file has been downloaded 824 times
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
@ Alice that was an interesting read...seems simpler than the method i was looking at. it's a bit difficult to obtain P2O5 though...and that was a
synth i wanted to try too. could you reduce P2O5 with carbon to obtain elemental P?
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
metal phosphides are sold commercially as rat bait, the phosphine being released in the gut after the metal phosphide is hydrolysed. Nice. Initially
they were released/sold without any coating but many a sheep were killed when the rain soaked the bait in the shed during a heavy storm, i believe
they are now only fatal if ingested not just wetted, that said i wonder if carrion eating animals fare poorly on the dead rats?
They are not sold at supermarkets and the like as they are generally used for heavy infestations, so rural product suppliers etc carry them in larger
quantities.
its a shame so much effort has be be gone through for such a ubiquitous element.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
according to UPS i should be receiving a package tomorrow so i will spend the day preparing the furnace and, if all goes well and weather allowing, i
will have some pics and a rundown of my process. upon reading an excerpt from an alchemical text, i have decided to somewhat prolong the process and
allow the necessary temp to be reached VERY gradually. a good preheat such as this should ensure dryness of the reactants and proper warming of the
delivery tube so as to avoid solidification of the distillate in the tube.
then again...arc furnaces don't pre-heat do they? i mean when you strike an arc with welding gear it is instantly HOT!
[Edited on 25-8-2010 by Rogeryermaw]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rogeryermaw  |
, i will have some pics and a rundown of my process. upon reading an excerpt from an alchemical text, i have decided to somewhat prolong the process
and allow the necessary temp to be reached VERY gradually. a good preheat such as this should ensure dryness of the reactants and proper warming of
the delivery tube so as to avoid solidification of the distillate in the tube.
then again...arc furnaces don't pre-heat do they? i mean when you strike an arc with welding gear it is instantly HOT!
[Edited on 25-8-2010 by Rogeryermaw] |
Very well, can't wait! During the run itself, put that camera aside: you want to keep your wits about and your legs in 'go, go, go!' mode in
the unlikely event things get hairy!
That Hennig Brand guy thought he was making gold from piss, I guess he got lucky not to get burned!
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
oh ya! its my birthday!! not really but every time i receive a chemical order i get giddy. i also picked up some refractory cement today so i could
make a furnace specifically for the preparation of phosphorus. i also purchased 2 lbs. of phosphate so i could run the synth multiple times if the
first run goes well. the time is close so wish me luck. and thanks for the advice blogfast. i have it in mind and i will be sure to take great care
during this process (limbering up for the hundred yard dash)
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Avoid suckback with an outlet shield
You can avoid suckback by putting a passive shield around the outlet pipe. The inert gas shield will exit out the top of the shield. A wier pipe with
integral trap keeps the water level low enough to avoid the outlet.
[Drawing rendered in Inkscape; original attached.]
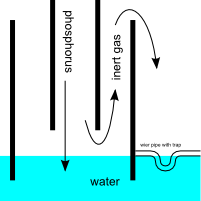
Attachment: P4 collector pipe.svg (8kB)
This file has been downloaded 886 times
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
sorry folks that its been so long. the week has been out of control! daughter started school this week. soon. promise.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
ok. i finished a preliminary run. i have come to the conclusion that i will have to switch to propane fuel to get the temp i need. the reactor
wouldn't get any hotter than a dull orange. according to info available from forge and foundry sources that's only 900-1000 celsius. also i may change
the reactor from rigid to emt. emt is steel but has a much thinner wall. the furnace is dug out in sand and the top is an old bbq lid coated with
refractory cement. it gets freakin hot but i think the emt will transfer more heat to the reactants. also propane will allow me to run it without
having to add fuel and lose heat. i will give it a go with the new ideas in place wednesday or thursday. thanks for being patient.
  
also wanting to give Al a try. the lower temp needed for reaction with aluminum is enticing.
[Edited on 30-8-2010 by Rogeryermaw]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Getting a higher temperature with propane will not be easy: provided you have plenty of air, lump BBQ charcoal burns like the clappers: very, very
hot! Your furnace looks very adequate to me. Is that incoming pipe for air?
What reagent mix were you using?
For Al, you can also use Al foil shredded in a food blender: sieve off the fines, that'll do in a good mix, preferably with metaphosphate (most Calgon
tablets - consult the labels). Fine Al shreddings, Na metaphosphate and SiO2 in the right ratio will work in your conditions, or I'll EAT MY HAT!
But please tell me that that isn't your condenser... you need at least something that's bottle shaped, IMHO...
[Edited on 30-8-2010 by blogfast25]
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
if you can show me a drawing or diagram of what you feel the condenser should entail please do. if i can see it i can build it. but the reason i opted
for propane is that during this first run i used charcoal and it burns away so fast that i have to keep opening the furnace to add fuel and it causes
me to lose a lot of heat. looking at some of the home foundry designs on youtube i see several designs running propane that actually melt steel to a
pourable temp. yes i know it is not the ideal mix but my reactants are calcium diphosphate, carbon, and silicon dioxide in stoichiometric ratios.
3CaH(PO4)+6C+3SiO2 -> 3Ca2SiO4+6CO+PH3+2P
is the balanced equation. using molar mass its roughly: CaH(PO4)=22.5g, C =4g, SiO2 =10g. and according to the eq. there IS some phosphine developed
so i will be staying well away when the reaction starts.
coming wednesday i will have a bit of cash to work on lab equip so i will get some Al powder and Na meta and have a go with it. it seems sodium
hexametaphosphate is readily available and relatively cheap here as photo chemical at about $14/lb
ya the pipe on the right is bringing air in from a blower motor. it is from a bounce house and moves A LOT of air.
seems an awful lot of trouble to get POCl3 which isn't even regulated. one of these days i'm going to start meeting meth cooks in dark alleys and beat
the hell out of them for ruining the future of research in america. i know its not entirely their fault but they are the scapegoat that gives the govt
and pharmaceutical companies fuel to take chemistry away from the rest of us... sorry for the rant but i know all of you feel the sting at one time or
another.
[Edited on 30-8-2010 by Rogeryermaw]
[Edited on 30-8-2010 by Rogeryermaw]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rogeryermaw  | if you can show me a drawing or diagram of what you feel the condenser should entail please do. if i can see it i can build it. but the reason i opted
for propane is that during this first run i used charcoal and it burns away so fast that i have to keep opening the furnace to add fuel and it causes
me to lose a lot of heat. looking at some of the home foundry designs on youtube i see several designs running propane that actually melt steel to a
pourable temp. yes i know it is not the ideal mix but my reactants are calcium diphosphate, carbon, and silicon dioxide in stoichiometric ratios.
3CaH(PO4)+6C+3SiO2 -> 3Ca2SiO4+6CO+PH3+2P
is the balanced equation. using molar mass its roughly: CaH(PO4)=22.5g, C =4g, SiO2 =10g. and according to the eq. there IS some phosphine developed
so i will be staying well away when the reaction starts.
coming wednesday i will have a bit of cash to work on lab equip so i will get some Al powder and Na meta and have a go with it. it seems sodium
hexametaphosphate is readily available and relatively cheap here as photo chemical at about $14/lb
ya the pipe on the right is bringing air in from a blower motor. it is from a bounce house and moves A LOT of air.
|
I've no design for a condenser but I was thinking of something like a vase with a more narrow top opening, half filled with iced water...
Actually, the famous painting of Hennig Brandt discovering phosphorus (instead of gold- hehehe) is an inspiration for a condenser...
http://2.bp.blogspot.com/_Bo_1OwpTPWg/Rxz-xOdD7fI/AAAAAAAAAB...
(Howzat for a diagram, eh!)
Charcoal fuelled furnaces do need regular refueling, that's true. It's the main drawback of mine too...
The chemistry however doesn't make an enormous amount of sense: why are you using the calcium diphosphate CaHPO4 and not the ternary tricalcium ortho
phosphate Ca3(PO4)2? Now you're planning to make PH3... ooops!
Your equation isn't balanced:
3 CaH(PO4) + 6C + 3 SiO2 -> 3 Ca2SiO4 + 6 CO + PH3 + 2P
... because it has 3 Ca on the left and 3 x 2 Ca = 6 Ca on the right. Correctly balanced it is:
2 CaHPO4 + 6 C + SiO2 --> Ca2SiO4 + 4/3 P + 2/3 PH3 + 6 CO
Note that the P/PH3 ration remains 2.
With Ca3(PO4)2 it becomes:
Ca3(PO4)2 + 5 C + 3/2 SiO2 --> 3/2 Ca2SiO4 + 2 P + 5 CO
... and no phosphine! For Ca3(PO4)2 you can basically use bone ash, or react your calcium diphosphate with slaked lime:
Ca(OH)2 + 2 CaHPO4 ---> Ca3(PO4)2 + 2 H2O
... then filter and dry on high heat.
Alternatively, go the sodium hexametaphosphate way... I believe you can even convert your calcium diphosphate into Ca metaphosphate and I think it's
explained in a post above.
Ooops.. it appears only Ca(H2PO4)2 can be easily converted to metaphosphate, not CaHPO4...
And after rereading much of this long thread, the more I read, the more I'm convinced the sodium hexametaphosphate/Al/SiO2 route is the way to go
because of the seemingly much lower temperature requirements:
2 NaPO3 + 4/3 Al + SiO2 ---> Na2SiO3 + 2 P + 2/3 Al2O3
(2 NaPO3 = 1/3 (NaPO3)6)
[Edited on 30-8-2010 by blogfast25]
[Edited on 30-8-2010 by blogfast25]
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
oh believe me i didn't want to be using the dibasic. i ordered bone ash and was sent bone ash synthetic. it's ok though. i like the metaphosphate idea
and will be using it. if i can find a decent smallish metal funnel i will weld it to the bottom of the delivery pipe. that should reduce the chances
of a vacuum induced catastrophy. you are correct about my equation. it should have looked like:
6 CaH(PO4) + 18 C + 3 SiO2 = 3 Ca2SiO4 + 18 CO + 2 PH3 + 4 P.
i hate to waste chemicals so i will play with these but i do very much like the idea of the aluminum reduction of the metaphosphate so i will run it
next. i also like the idea of eliminating PH3 since it's deadly and a waste of phosphorus.your helpfulness is much appreciated. particularly the lack
of venom. far too much anger and biting sarcasm find there way here and you conduct yourself like a professional. thank you.
BTW i do so love this picture!
[Edited on 30-8-2010 by Rogeryermaw]
[Edited on 30-8-2010 by Rogeryermaw]
|
|
|
aliced25
Hazard to Others
  
Posts: 262
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
The type(s) of condensers are enormous, but when working with such temperatures you'll need a working fluid (and fins for heat transfer) inside an
outer pipe, with the working fluid being pumped in at the bottom and out at the top. One of the glycols should be up to the task. I'd suggest cutting
some fins into the inner pipe ABOVE where the outer pipe joins it, so it works as an air-condenser, precooling the distillate before it gets to the
condenser.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aliced25  | | The type(s) of condensers are enormous, but when working with such temperatures you'll need a working fluid (and fins for heat transfer) inside an
outer pipe, with the working fluid being pumped in at the bottom and out at the top. One of the glycols should be up to the task. I'd suggest cutting
some fins into the inner pipe ABOVE where the outer pipe joins it, so it works as an air-condenser, precooling the distillate before it gets to the
condenser. |
I don't think anything like that is necessary. While the painting isn't necessarily technically very accurate, Brandt achieved reduction of phosphate
in primitive conditions, without sophisticated condensers. Brandt was eventually reduced to selling the method to various buyers (see p. 312, of the
excellent 'Nature's Building Blocks' by John Emsley).
Others like me have actually pointed out to the risk of the P cooling and solidifying TOO QUICKLY! We have some evidence of that happening in
experimental conditions, higher up in the thread...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ Roger:
It is a wonderful painting. He (they?) must have been flabbergasted, having made the first glow-in-the-dark, instead of gold...
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
going by info i collected from my last attempt, after about 25-30 minutes in the fire, the vessel heated the output to a fairly warm temp. well within
the liquid range for phosphorus. an idea i have come up with is to weld a stainless funnel such as this to the end of the delivery tube. the increase
in area at the opening should make it difficult for the reactor to pull in enough water to do damage in that if it pulls water in to a certain volume
the end of the funnel would no longer be submerged thus breaking vacuum. any thoughts?

|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Getting to within the liquid range of P isn't near good enough, of course: you need to get the reaction going which in the case of orthophosphates
going by he literature may well be above 1000 C.
Your funnel idea seems good to me. Not sure it's really necessary; you'll have to see...
I think I might have another open crucible test with NaPO3/Al/SiO2 for some better observations...
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i'm sorry i may have been unclear. the reaction vessel was quite hot. the section i was referring to was the part outside of the furnace. it was
uncomfortably hot to the touch so no worry about P4 solidifying in the tube. also i boiled the water in the catch pan before placing it under the
tube.
[Edited on 31-8-2010 by Rogeryermaw]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yes, I misunderstood you. I think you're right.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
tomorrow or thursday i will run it again with the lp gas. i will also order the sodium metaphosophate. i may use the furnace to melt my own Al. i want
to try the idea i found about stirring molten Al to produce coarse powdered Al. be nice if it was suitable for this reaction.
|
|
|
| Pages:
1
..
20
21
22
23
24
..
60 |