| Pages:
1
..
20
21
22
23
24
..
33 |
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Quote: Originally posted by blogfast25  | | 'Conc.' is a bit of a historical misnomer, I'm afraid. Practically it means the highest concentration that an acid can be obtained as with respect to
water as a solvent (or remainder or impurity). |
Sort of helpful but I think it kind of confused me, the part where you say "can be worked up to about 100 w%". Does this mean no water at all or the
most that water will hold. I often use these charts:
http://www.sigmaaldrich.com/chemistry/stockroom-reagents/lea...
http://www.csudh.edu/oliver/chemdata/acid-str.htm
Where Nitric is 70.4, Sulfuric is 96.0 as example. My problem stems from over years only studying enough chemistry for specific things I was working
on at the time. Reading this thread I can see catching up is a long way off. What I was trying to figure out was what they on average mean in terms of
percentage when they say 'concentrated'. I know I am kind of going off your real topic but when you talked about HCL percentage I figured it was a
chance to jump in and get an answer to something that has always given me trouble. Taking the quote from:
https://en.wikipedia.org/wiki/Nitric_acid
"Commercially available nitric acid is an azeotrope with water at a concentration of 68% HNO3, which is the ordinary concentrated nitric acid of
commerce.
Nitric acid of commercial interest usually consists of the maximum boiling azeotrope of nitric acid and water, which is approximately 68% HNO3,
(approx. 15 molar). This is considered concentrated or technical grade, while reagent grades are specified at 70% HNO3."
and
"A commercial grade of fuming nitric acid contains 90% HNO3"
When they say concentrated (using Nitric as my example) I never know do they mean 68%, or 90%. I was trying to make flash paper once that didn't work
at all and what I had was the typical 68% that a supply house in Phoenix carried yet the bottle had concentrated on the label. So I guess what my real
question is, how do you tell in a procedure when they state concentrated but fail to mention numbers. I ask because this failure to specify is
incredibly common in many things I have read. I won't go further as I do not wish to get you too far off the topic of 'wave mechanics in chemistry'
asking something better suited for beginnings, but this question has really bothered me for years.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The vagueness of 'conc', 'hot', 'cold' could easily balls up the experiment (especially with me doing it !).
Nobody needs panic about aga and acid, as i have some Warm, Dilute NaOH on hand ...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
IrC:
100 w% here means pure acid, no water.
Sulphuric acid goes up to 98 w% which is the azeotropic concentration. But 96 and 95 w% are routinely sold and referred to as 'conc. H2SO4' too. And
Oleum is kind of 200 %: it's anhydrous H2S2O7 (a solution of 1 mol SO3 in 1 mol H2SO4) but rarely available commercially.
Nitric acid is sold often as azeotropic NA, at 68 w% but I've seen the term used also for 75 w%! And > 95 w%, so called fuming nitric acid, is also
available. 'White fuming nitric acid' is 99.9 w%.
Whatever the acid used, technical and academic papers should ALWAYS specify actual concentration, sadly they don't always.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | The vagueness of 'conc', 'hot', 'cold' could easily balls up the experiment (especially with me doing it !).
Nobody needs panic about aga and acid, as i have some Warm, Dilute NaOH on hand ... |
Brilliant! A recipe for even deeper burns! Heat of the NaOH solution + solvation heat of acid + neutralisation enthalpy + (in some cases) lattice
energy of formed salt = skin graft!
Hint: best practice for most acid spills on skin: treat with copious amounts of cold, fast flowing tap water. Simples...
[Edited on 28-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Imagining is one thing !
Spent hours trying to make them work in terms of orbitals & charges and none of the 'imaginings' can physically work.
Makes great doodles though.
Came across (and tried to apply) Lewis Dot Diagrams in the process of proving myself wrong.
'twas merely jest, sirrah.
Naturally one wears an all-in-one bullet proof rubber incontinence suit when handling acids.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'm quite curious to know what Darkstar thinks of mine, actually...
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@aga
If it helps, here's blogfast's mechanism with arrow pushing and possible intermediates. Like before, H–A represents the acid catalyst and
A<sup>–</sup> is its conjugate base. Unfortunately, I'm a little busy with school work at the moment, so I can't do a step-by-step
analysis of the mechanism right now.
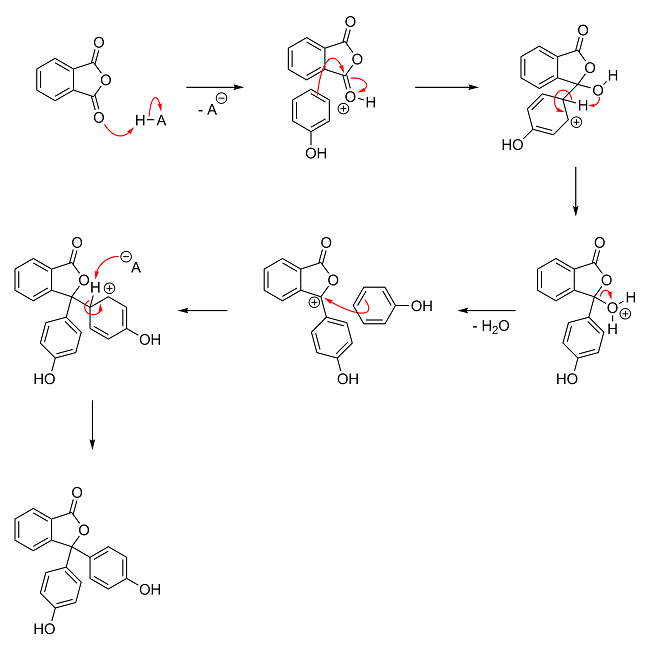
Also, if you're interested, here's the mechanism in its original format and resolution:
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Beautiful Darkstar ! Thanks.
The mechanism is so far removed from anything i could think of, one would expect to disappointed.
The Opposite is true !
It's so amazing to be able to even Attempt stuff like this.
(i think he likes it bloggers. I reckon you dropped 2 marks: one for omitting the curly arrows, the other for eschewing the snakes-n-ladders format)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | I reckon you dropped 2 marks: one for omitting the curly arrows, the other for eschewing the snakes-n-ladders format) |
Aaaarrrghgh!!! Rarely has my honour been besmirched more than on this wretched day... You leave me no choice Sir but to tear all my hair out.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Keep your hair on.
2 marks were deducted, so 8/10
Then the extra marks for starting/teaching the thread were added, so 998/10.
9980% is a pretty good result.
Edit:
10 marks were deducted from the max 1000 available for the Thread Award due to some drunkard being allowed on the course.
[Edited on 28-11-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
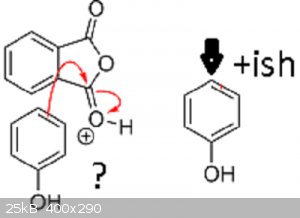
Once again, please forgive me if this is something i've simply not understood properly.
Surely an electron transfer imparts an increase in -ve not +ve, so i can safely assume that the '+' in the circle should be a '-' (i.e. a typo)
For the Phenol, would the electronegativity of the O in the OH group Not pull electron density towards it, creating a slight nett +ve charge on the
opposite carbon of the benzene ringy bit ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Once again, please forgive me if this is something i've simply not understood properly.
Surely an electron transfer imparts an increase in -ve not +ve, so i can safely assume that the '+' in the circle should be a '-' (i.e. a typo)
For the Phenol, would the electronegativity of the O in the OH group Not pull electron density towards it, creating a slight nett +ve charge on the
opposite carbon of the benzene ringy bit ?
|
I'm not enamoured with that bit of Darkstar's rendition either and prefer mine.
Note that -(-) = +: take away a negative (from zero) and you end up with a positive.
As regards you last paragraph, it sure remains a little mysterious as to why the C opposite the C-OH is the attacker here, no contest from me. Do bear
in mind that the electron rich (and delocalised) π rings are quite nucleophilic and suitable for attack on a carbocation.
Steric hindrance may have something to do with the 'choice' of carbon No 4 because it's the most accessible of all.
The electronegative O does make the whole ring slightly less negative, correct.
Oh, and look at the way Darkstar wrote the first step of the esterification reaction (higher up), which is also a protonation step:
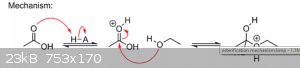
So he prefers to write it that way but when one of these C=O-H (double) orbitals moves, then the positive charge moves onto the C. And it's the
cabocation that's crucial here because it's so electrophilic! So I prefer my simpler notation (nerner nenenener!) 
I'm sure the occasional murder has been the result of disputes about OC mechanisms!
[Edited on 29-11-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I leave for a couple of hours and this is what I come back to!? Alright, let me 'splain.
Please, by all means, keep asking questions. This is how you learn.
| Quote: | | Surely an electron transfer imparts an increase in -ve not +ve, so i can safely assume that the '+' in the circle should be a '-' (i.e. a typo)
|
Your eyes deceive you not. There's supposed to be a + in that circle. The positive charge is on phthalic anhydride's carbonyl oxygen, which got
protonated by the acid. I always draw activated carbonyl groups like that. I discussed what "activated" means in detail in my Fischer esterification post.
| Quote: | | For the Phenol, would the electronegativity of the O in the OH group Not pull electron density towards it, creating a slight nett +ve charge on the
opposite carbon of the benzene ringy bit ? |
It's actually the exact opposite. If oxygen's lone pair drops into the aromatic ring and becomes delocalized, the meta-directing inductive effect
(pull of oxygen) that would normally direct electrophilic attacks to the 3-carbon (meta) will get over powered. If you were to draw the resonance
structures, you would see that phenol's oxygen has a lone pair that can be donated into the benzene ring, which creates a negative charge at the
2-position (ortho) and 4-position (para). Thus phenol's -OH group is actually a rather good electron-donating group and strongly activates the ring
for electrophilic attack at the 2- and 4-position (called ortho-/para-directing).
Inductive effects are not always the most significant contributor. Always take into consideration resonance if applicable.
Quote: Originally posted by blogfast25  | As regards you last paragraph, it sure remains a little mysterious as to why the C opposite the C-OH is the attacker here, no contest from me. Do bear
in mind that the electron rich (and delocalised) π rings are quite nucleophilic and suitable for attack on a carbocation.
Steric hindrance may have something to do with the 'choice' of carbon No 4 because it's the most accessible of all. |
I think it's a combination of steric hindrance and phenol's strong ortho-/para-directing effect (see above). If the electrophilic attack happened at
the meta-position, the oxygen would be unable to donate a lone pair into the ring to form an oxonium ion and stabilize the intermediate. This makes
the ortho- and para-positions much more favorable. And since the ortho-position is physically blocked by the -OH group, the para-position ends up
being the more favorable of the two.
| Quote: | So he prefers to write it that way but when one of these C=O-H (double) orbitals moves, then the positive charge moves onto the C. And it's the
cabocation that's crucial here because it's so electrophilic! So I prefer my simpler notation (nerner nenenener!)  |
Technically, your way is no more correct than mine! I draw it all in a single step (nucleophilic attack and pi-bond cleavage) mostly because it saves
a step. But in reality, the positive charge is neither completely on the oxygen nor completely on the carbon. So whether you draw it like I do with
the charge on the oxygen, or like you do with the charge on the carbon, in reality it's on both.
I do think it helps beginners to see the charge on carbon so they understand why it gets attacked by the nucleophile, though; however, these days I
just put the charge on oxygen and imagine it as oxygen pulling harder on carbon because that extra proton is now pulling on it (i.e. the inductive
effect). In other words, the extra proton causes oxygen to pull even more electron density away from carbon than it normally does, activating it for
nucleophilic attack.
[Edited on 11-29-2015 by Darkstar]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks for the clarifications Darkstar, especially the influence of the oxygen on the delocalised π ring in phenol, my bad on that one. And just
after explaining why phenol is a weak acid too! Tsk... 
Coming up next: the Markovnikov Effect!
[Edited on 29-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Before explaining the Markovnikov effect, I want to go back to my proposed mechanism for the synthesis of indole-3-acetic acid, summarised here:
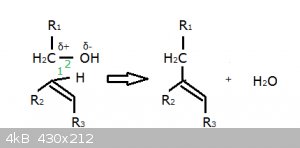
At the time no one cottoned on to the question ‘why does the addition occur on C No3 and not on the one below’?
Well, let’s look at the full structure of indole:

The No3 C is closer to the aromatic π ring and some orbital overlap increases the electron density (an effect sometimes described as ‘electron
pushing’) on the No3 C, making it more susceptible to nucleophilic attack than the one below it.
We do well to remember that the neat little bars that represent MOs in our various notations create the illusion that all MOs are equal but due to
various effects this is not true: due to differences in electronegativity and/or resonance/orbital overlapping, and these effects can determine
outcomes significantly.
The Markovnikov Effect:
Let’s look at the electrophilic addition of HBr to 1-butene:
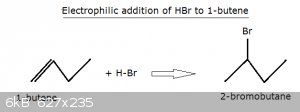
We’ve already covered this type of addition higher up.
In essence the highly polarised H-Br bond splits into a proton and a bromide ion and the proton snatches the π orbital and ends up on the No1 carbon.
That leaves the No2 carbon as a carbocation. The bromide ion Lewis base then shares one of its (four) lone electron pairs with the carbocation et
voila, 2-bromopropane is born!
However, this begs the question, ‘why does the carbocation not form on the No1 carbon?’, which would give rise to 1-bromopropane?
The reason is that the No2 carbocarbon is bound to an ethyl group (-C<sub>2</sub>H<sub>5</sub> and a methyl group which are both ‘electron pushers’, whereas the No 1 carbocarbon
is only bound to two hydrogen atoms and one propyl group. The electron pushing effect of the ethyl and methyl group lowers the charge and thus the
energy of a carbocation on No2 carbon somewhat and this is preferred to a carbocation on the No1 C. and a methyl group which are both ‘electron pushers’, whereas the No 1 carbocarbon
is only bound to two hydrogen atoms and one propyl group. The electron pushing effect of the ethyl and methyl group lowers the charge and thus the
energy of a carbocation on No2 carbon somewhat and this is preferred to a carbocation on the No1 C.
The Markovnikov effect can thus be summarised as follows:
If there is a choice of carbocations to be formed, the carbon atom bound to the most/strongest electron pushers is the preferred one. We can even
simplify further: the carbocation forms preferably on the carbon atom that’s bound to the higher number of other carbon atoms.
[Edited on 30-11-2015 by blogfast25]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Blogfast, aren't you referring to the <i>Markovnikov</i> effect? I've never heard of this phenomenon referred to as Markovsky effect.
[Edited on 11-29-2015 by gdflp]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
gdflp could be right Prof.
Seeing as he's not enrolled on this course i called Security and had him thrown out of the building.
We can cover it up no problem - nobody will ever know, just alter the records and it will all be OK.
[Edited on 29-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Old age, poverty and fading memory: it's been corrected.
Thanks gdflp and aga.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Erm, there's a slight problem.
Security immediately did as asked.
Thing is, they're not too bright, and we're on the Fourth floor, and gdflp turns out to be the Bursar ...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Erm, there's a slight problem.
Security immediately did as asked.
Thing is, they're not too bright, and we're on the Fourth floor, and gdflp turns out to be the Bursar ... |
Give him a large mince pie. T'is the season of good will, shortly.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It was mice pies that saved the day.
The bio-chem testing lab had something of a surplus of ex-test-subjects and decided to team up with class 6C doing food science, and made a very large
number of pies.
The lab should really have mentioned the Substances they were testing ...
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Blogfast and Darkstar are doing an excellent job of explaining theoretical organic chemistry, but so far there has been no discussion of practical
organic chemistry such as extractions, explanation of fractional distillation and vapor phase diagrams, etc.(unless I missed it). Would you like me
to jump in and contribute several sections on these topics aga, as they're quite important in organic chemistry? They're also important in getting
high proof alcohol, which I know you're interested in I can
also throw in some labs based on the theory if you'd like. I can
also throw in some labs based on the theory if you'd like.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I think your Input would be extremely valuable gdflp.
If blogfast25 and Darkstar have no objections, please join the Party !
(Do not expect too much of your student(s) though)
Edit:
For the Record : i drink only Beer <= 4.5w%
More than that and i go all nuts.
Distilling ethanol was interesting to prove/disprove blogger's Thumper question.
Near 100% EtOH was interesting when deltaH's K3PO4 idea worked out well.
To distill and Drink is not one of my hobbies.
Perhaps that's one reason why homedistiller.org didn't like what i said (at all).
[Edited on 29-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | I think your Input would be extremely valuable gdflp.
If blogfast25 and Darkstar have no objections, please join the Party !
|
Sure, no objections!  In fact, it's much welcomed from my side. In fact, it's much welcomed from my side.
[Edited on 30-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Primary, Secondary and Tertiary Carbon Atoms:
In the spirit of Markovnikov's Rule, look at the following hydrocarbon compounds in which an exo-atom (or some group) X replaces a hydrogen atom:
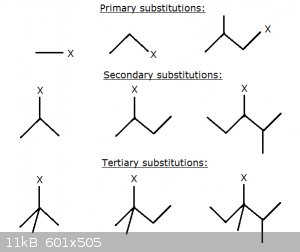
In the top row, the C to which the X is bound is itself only itself bound to zero or one other C. We call that C a primary carbon atom.
In the middle row, the C to which the X is bound is itself only bound to two other C. We call that C a secondary carbon atom.
In the bottom row, the C to which the X is bound is itself bound to three other C. We call that C a tertiary carbon atom.
Note that in all three classifications the nature of the groups bonded to the C atom in question is of no importance: they may be long, short, contain
double bonds (but not to that C atom itself) or even exo-atoms or functional groups without affecting its classification.
In general, in analogy with Markovnikov's rule, we can say that the electron density about the C-atom will vary broadly as: Tertiary >
Secondary > Primary.
And this, together with steric hindrance considerations, has consequences, in particular for nucleophilic substitution reactions (next up!)
[Edited on 30-11-2015 by blogfast25]
|
|
|
| Pages:
1
..
20
21
22
23
24
..
33 |