| Pages:
1
..
19
20
21
22
23
..
48 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Gold, alas is no good
Thanks for that Sedit,
O well, it's good to clear it up. As someone used to say in my neck of the woods:
"Thinking thought your bum was Castletown" 
(Sorry it's a bit rude!!)
I think the Diamonds are some way off for us, perhaps not.
There would be nothing to beat a bit of bling when it come to Chlorate and Perchlorate making!
A good anode for to make Chlorate is the Manganese Dioxide anode. It will last a very long time in a pH controlled cell IMO. It has only been tried in
a non pH controlled cell where is lasted some weeks, though the cell went a rather, shall we call it, gay colour.
Cheers,
Dann2
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
"Thinking thought your bum was Castletown"
(Sorry it's a bit rude!!)

Yehhhh... I THINK I may have been confusing some of the gold perchlorite solutions
they used for electrolyts in some papers with gold electrodes. I may have been confusing some of the gold perchlorite solutions
they used for electrolyts in some papers with gold electrodes.
Oh well happends to the best of us. Either way my statement on using gold leaf to cover carbon electrodes still holds true and I have used them for
various applications because of the low corrosion rate and cheap way to gold electrodes.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by dann2
Chromium also buffers the cell. I am not using any.
From the pH controlled Graphite anode cell I have good news and weird news.
First the weird.
I was adding acid at quite a large rate, as stated above, and increasing the rate so to get pH down to 6.8. The rate had gone up to 0.42ml per hour
per amp. The pH dropped to 6.7 so I decided to turn off the acid to let pH increase a bit. I assumed that when acid was turned off for about an hour
or so the pH would go back up.
24 hours later with no acid going into cell the pH had increased from 6.7 to 6.8!!
The cell seems to be staying rock steady at the wanted pH without adding any acid. |
That IS good news, and verifies something I noted earlier... with NO acid additions to a newly-fired up chlorate cell, the pH seems to rapidly climb
to 8 or 9+, and stays there... we are into the "9-electron" mode of chlorate production. Call it Mode 9, for lack of a better term.
Then, the acid goes in, and you enter "Mode-6". There seems to be some sort of natural buffering that takes place. Additional acid has less of an
effect than one might suppose, and the pH does NOT tend to climb as badly as it does when the cell is first energized. Weird, but beneficial.
Implication: An initial control of pH is essential, acid is needed, but once "in the zone", the system tends to stabilize nicely, with only
occasional checks of the pH needed.
| Quote: | The temperature of the cell is low at 23C so conditions are not optimum for 'Chemical Chlorate formation'. I guess I could wrap some insulation around
it but am not going to bother. Will just let it run its course (approx 5 days).
|
I think your low temps are keeping the graphite intact, and the process clean, at the expense of a bit of efficiency - the former being preferable to
the latter, unless the efficiency is now down to 8% or something ridiculous. You'll find out soon enough! Is there enough liquor to draw off a
sample and do a quantitative analysis of the chlorate concentration? And are you tracking chloride as well? Those Hach chloride titration strips I
mentioned are handy as hell, although at a price. At 85 cents per strip, it's still probably cheaper than mixing your own silver nitrate titration
solution; easier too.
| Quote: | Thats another advantage with using MMO when controlling cell pH in a one cell system. You can let the temperatrue go way up to 70C or so and since MMO
is so tough it does not seem to get damaged. 70C would probably erode Graphite too much.
To get the best from Graphite you would really need a two compartment system. One compartment with anode (below 35C or so) and another larger
compartment with a higher temperature (70 - 80C) for to achieve optimum conditions for the 'Chemical Chlorate formation' to take place.
|
That's where my old "Super T-Cell" would have shined, if I had started with sodium salts. Something I still want to try. By varying the speed of the
peristaltic pump, I was easily able to get a Delta Temp. of 40 to 50 degrees C. Of course, using graphite, you'd be in a sort of "reverse" mode...
you want to keep the electrolytic cell (w/anodes) cool (immerse cell in H2O and blow a fan across the water) and add heat to the bulk cell for
chlorate formation.
| Quote: | I think the lesson to be learned from this cell is that if you want to avoid the mess with Graphite and have the anode last long and long then
controll the pH. iT's early days yet and the anode may start to erode after some time.
Dann2 |
Are you tracking voltage and current? I hope it's not too early for a congrats on a graphite cell that is clean... hopefully it'll stay that way.
Nice job!
Swede 
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I am tracking current (it stays steady at approx. 5.2 amps.) but not voltage. I will start measuring it.
The supply is a computer PSU with Nicrome wire resistor.
After five days the solution is <strike>Crystal</strike> Diamond clear!
If you rub your finger on the anode black comes off.
The amount of acid keeping the pH at 6.9 is now a sensible amount (0.115ml per hour per amp, that 0.6ml per hour going into cell).
I am taking a sample very day so will titrate and get an idea of CE etc. Have no Chloride strips, too miserable to purchase though they would
definitely be very very handy.
The cell contains 2 liters of saturated Na Chloride so it should be coming near the end of it's 'run time'.
Will let it run untill erosion starts to show on anode and that will tell me what the Chloride concentration is at the point where erosion starts.
Perhaps there will not be a sudden start to erosiong due to low Chloride.
The picture below shows the end of the anode. The corner not in the solution can be seen and it is still sharp like when it started off. The other
corner looks eroded a bit, but thats because it is under the solution surface and bubbling, giving visual distortion.
@Swede, have you cranked up a (4 month long run) cell to test the LD on MMO'ed Ti yet????????????????? 
Dann 2
LONG LIVE THE POOR MAN'S MMO!
[Edited on 29-1-2009 by dann2]
[Edited on 30-1-2009 by dann2]

|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Looking good Dann2! Historically, how low can you go (Cl-) with graphite anodes before erosion becomes a real problem? Once the efficiency of the
setup is known, you can certainly predict chloride vs. chlorate with relative accuracy. Good to hear that the HCl dosing is working as well. At 6.9
pH, you are right at that sweet spot for chlorate formation. I'll bet the efficiency will be fairly high.
I haven't cranked up the LD anode yet. I wanted to make one or two more, and the WX has been too cold. I stopped by a local "Container Store", and
if you don't mind paying the $$, (and are in the U.S.) they have quite a bit of Nalgene lab quality bottles and other useful stuff for a lab. Anyway,
I picked up some small (2 liter ) PET plastic food containers for test purposes.
Latest business has been recrystallization of some Pt-produced perchlorate. Along with sodium sulfite and KOH to neutralize, I hope to have
chlorate-free perc when the smoke clears. No point in making perc for pyrotechnics if you can't clean it adequately. Anyone have any idea on how
industry uses Sodium Sulfite to reduce chlorate ions? I took a guess and added 4 grams to 300 grams of boiling perchlorate. It's going to suck if it
takes multiple recrystallizations to remove all chlorate. I'd like to avoid using acid if I can.
Edit to add: The reason I wondered about voltage was because I was not sure about your power supply, if it was a constant current supply or not.
With CC, you can get interesting (and I think valuable) data from the voltage as it varies. Plotting voltage vs. time produces a curve that is
predictable, and I think it could be useful to calculate end-of-run conditions.
[Edited on 29-1-2009 by Swede]
|
|
|
hashashan
Hazard to Others
  
Posts: 255
Registered: 10-10-2006
Member Is Offline
Mood: No Mood
|
|
Its been some time,
now since i work in a plasma lab I have acess to some interesting things.
Did anyone try conductive Zinc oxide plated on glass?
anyone knows how well will it last in the harsh cell conditions?
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Hashashan,
By coincidence there is a paper on Al doped Zn Oxide films over in the reference section. See post of posted on 28-1-2009 at 08:51 PM
I have not read it and don't know if it is of any use or not.
Cheers,
Dann2
|
|
|
hashashan
Hazard to Others
  
Posts: 255
Registered: 10-10-2006
Member Is Offline
Mood: No Mood
|
|
I think Ill just try that out, in a completely uncontrolled system and se when it will fail.
Ill start the run on monday
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I made a valiant effort to remove traces of chlorate from a modest batch of Pt-generated perchlorate and blogged the results here.
It didn't work. I think I made an error by neutralizing (actually probably more like pH 8) prior to adding the sulfite. If the solution is not
acidic, SO2 is not produced, and little or no reduction takes place.
Next cleanup attempts will consist of potassium metabisulfite on a smaller scale.
Another LD plating will take place, hopefully tomorrow, with the goal being more of a flash plating than a semi-massive LD anode. If a light plating
adheres to MMO, AND produces perc successfully, then I'll count it as a success. I am going to repeat the process that produced the better anode,
especially the heavy boiling of the anode in the surfactant solution.
I also came up with yet another heater idea... I have a bit of Ti tube... crimp and/or weld shut, install the cartridge heater, and either surround
the heater with fine grog, or wrap with 304 SS foil for a tight, sliding fit in the Ti tube.
[Edited on 4-2-2009 by Swede]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
I also came up with yet another heater idea... I have a bit of Ti tube... crimp and/or weld shut, install the cartridge heater, and either surround
the heater with fine grog, or wrap with 304 SS foil for a tight, sliding fit in the Ti tube. |
If you've got
some of one of those low melting point bismuth alloys, you could cast it in place. Reversible in hot water.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by watson.fawkes
| Quote: | Originally posted by Swede
I also came up with yet another heater idea... I have a bit of Ti tube... crimp and/or weld shut, install the cartridge heater, and either surround
the heater with fine grog, or wrap with 304 SS foil for a tight, sliding fit in the Ti tube. |
If you've got
some of one of those low melting point bismuth alloys, you could cast it in place. Reversible in hot water. |
I'm afraid most of the cheap cartridge heaters have wattage densities high enough to liquify the alloy even with the outer tube immersed... but a
castable metal (perhaps lead?) is a great idea.
I had some success cleaning up perchlorate with sulfite, but it took more sulfite than I expected, indicating that my perc was exceptionally dirty, or
my method sucked. It required 4 grams of sulfite to clean up 20 grams of perchlorate.
Any thoughts on perc purification? Selective or preferential reduction of chlorate vs perchlorate is a bit of a mystery... why does the chlorate
reduce, but not the perchlorate?
Googling any combination of "purification of perchlorate" or "reduction of chlorates with sulfites" yields either groundwater purification processes,
or bacterial investigations.
So far, my technique (for now) consists of bringing to a boil a near-saturated solution of raw perc, acidifying with HCl, then adding sulfite salt in
measured quantities, liberating the reducing agent SO2. The addition of the sulfite salts increases pH dramatically, and my modest research indicates
the solution must be acidic to work correctly, thus fairly large amounts of HCl are required. After testing clean, the perc (for pyrotechnic use) is
neutrlized with KOH, chilled, and collected.
I mentioned to Tentacles in a PM (he already has a sheet) - I bought three sheets of that angled MMO from the eBay guy at $10 per sheet. Odd stuff.
Definitely MMO. The underlying Ti is thicker than my stuff. The MMO appears to be a different comp... the shade is very slightly different; OR, it may
be used, which is no big deal because the stuff doesn't get used up, really.
The 90-degree part looks to be browner than the rest, and I believe this stuff was used in a chlorine plant or water purification plant, whereby the
electrical connections are made by clamping the bent part in big clamps, while the remainder did the work. Just a guess.
I'll throw a shank on one and try a test. I bet it'll work (and plate) fine.
The price at $10/sheet is cheaper per square inch than the stuff I bought earlier... might be worth grabbing one or two before they're all gone:
MMO Sheet at $10 per
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
The Perchlorate ion is very stable, that is why it persists in the environment for so long. The Chlorate ion is less stable and easier to reduce.
edit:
There is a reaction equation here for Metabisulphite.
and some stuff on Sulphite/Bisulphite dissociation here
You could consider doing a titration on your sample (before Chlorate destruction step) to see how much actual Chlorate is in it. It's not too
difficult to do using Ferrous Sulphate, Potassium Permanganate, Manganese Sulphate (not too sure what the Manganese Sulphate is for) and Sulphuric
acid + some time.
+ another edit>
Mix some of your product with sugar and drop conc. Sulphuric acid on it. If you get ignition (or crackling noise) you have far too much Chlorate to
start destroying with chemicles.
Dann2
[Edited on 6-2-2009 by dann2]
[Edited on 7-2-2009 by dann2]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
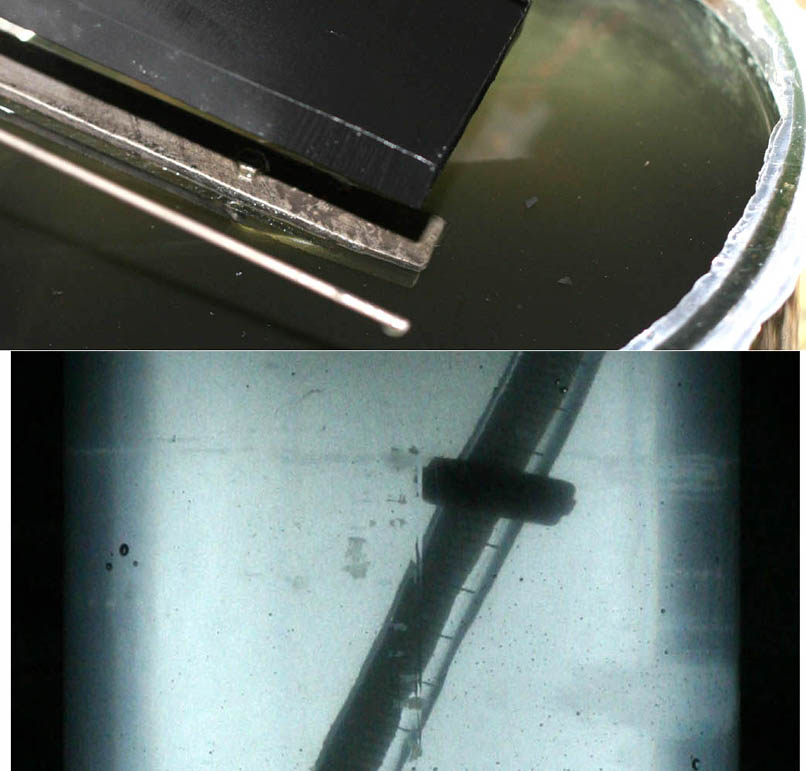
Some pictures of Graphite Anode from a pH controlled cell. Erosion is low. It is now day 14.
Up a few posts I stated the 'run time' of this cell was about 5 days which was totally wrong. The cell contains approx. 2 liters. Assuming 320grams
per liter NaCl (11 moles total NaCl) and I convert all the Chloride into Chlorate at 100% CE then at 5.5 amps that is going to take 13.5 days. If I
leave 100 grams per liter Chloride and get 70% CE it will take 14 days.
Anyhow I am taking samples and we shall see later.
You could probable use KClO3 directly from this cell as there would be very little black erosion product.
The solution is not clear. The picture with the thermometer in a jamjar with cell solution shows no black at all while the other picture exaggerates
the black hue too much in the solution.
There is very little erosion to be seen on the anode. I intend to let the cell run untill there is alot of erosion on the anode which (I presume) will
start to occur when Chloride gets low. Cell temperature is 18C.
Dann2
[Edited on 7-2-2009 by dann2]
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
| Quote: |
The price at $10/sheet is cheaper per square inch than the stuff I bought earlier... might be worth grabbing one or two before they're all gone:
|
@ swede-Thank you for the link. Any luck with this? Let us no
@dann2 and anyone else interested:
I found a seller who is selling sodium metabisulfite and other chems at good prices. Just click here
[Edited on 2/7/2009 by chloric1]
Fellow molecular manipulator
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
those cheap sheets do seem pretty scratched up tho
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
Thanks Chloric1. I don't actually make much Chlorate/Perchlorate. Testing anodes mostly. I would be inclined to try Ferrous Sulphate for destroying
Chlorate. I can get tons of it cheap.
The Titanium mesh (with the MMO coating, but nobody is 100% sure yet, variety) is cheap even if there is no MMO coating on it. Ti mesh is hard to come
by (unless you happen to be willing to 'visit' and 'charm' the pool chlorination community........) ;-)
I will not be convinced about the (free) MMO coating on the Ti mesh untill someone sticks it into a Chlorate cell. Colour alone is not an indication
of a coating that will make Chlorate! Hope I am wrong though.
Dann2
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
well that will be me in the next day or two. i may take some pictures. And have some Chlorate/No Clorate results in a week or so.
what math do you guys use for purity analysis?
i would say:
heat a measured amount
calculate theoretical O2 loss
and divide the measured loss by theoretical
That's assuming a KCl/KClO3 only mixture which is(?) an ok assumption.
Do i need a catalyst?
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by y2kbugger
those cheap sheets do seem pretty scratched up tho |
The ones I got seemed pretty decent. There were one or two locations where the MMO is thin or rubbed off, but I'd say the sheets I bought are 99%
covered, and they look good physically. You might have gotten a poor example.
Exposed Ti is not a problem. So long as the MMO does not detach from the Ti, and the formulation is correct, I think it will make chlorate just fine,
and exposed Ti will simply oxidize. The cut sheet I've been using from the start has exposed Ti at the edge, and the hanger strap (shank) itself is
also exposed and immersed with no problems at all.
I've cut off the 90-degree part and am now wondering what to do with it. Make mini-anodes? Salvage the MMO in some fashion, mechanically or
chemically? A while back, I did try dipping a scrap of commercial sheet into cold, 70% nitric with no apparent reaction. It seems to be tough stuff.
Hint for cutting - Use a vertical bandsaw with as fine a teeth set as possible, and run it as slowly as possible. Back the mesh with either very thin
plywood, or thick cardboard. It cuts nicely in this way. I'd wear a dust mask, but I doubt very much that MMO is being ejected or becoming airborne,
but better safe than sorry.
Edit to add: y2kbugger, if the MMO is capable of making chlorate, then to answer your question, no, I don't think any added catalyst is needed or
desirable. If I was forced to choose one, I'd prefer NaF over dichromate, as you have a non-cumulative, vs. a cumulative poison. The NaF should
easily remain dissolved in either the wash of the K-crystals, or the metathesis to potassium salts of your Na-based system.
[Edited on 8-2-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@Swede You can weld the scraps together with you spot welder. If you weld an ordinary piece of Ti to a strip you could make an anode with all the MMO
(if it is MMO) underneath the surface. The piece that you have cut off is an inch wide. Not very small at all at all.
You could also use them as small (desirable) Cathodes with larger anodes. .
Dip the end of one of them into a salt solution quick with power connected and relieve us of the suspence/anticipation.
Heating to drive off Oxygen should work OK. You will need an accurate scale of the amounts of Chlorate are small. Make sure it is dry before you
start. I think Manganese Dioxide acts as a catalyst for the decomposition of Chlorates when heated.
And replying to post below!
Would a pair of wire snips not cut the mesh?
Dann2
[Edited on 8-2-2009 by dann2]
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
so is the oxide coating non conductive then? like what if one were to use just a sheet of titanium?
ha i used a dremel, went through 3 cutting disks before i got two electrodes made.
[Edited on 8-2-2009 by y2kbugger]
replying back up to ya, I tried some very large tin snips and that didn't work, and I didn't wanna risk chewin up my wire cutters.
[Edited on 8-2-2009 by y2kbugger]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
It must be Grade 2, 3, 4 or 5 stuff.
It definitely is not Grade one as it is quite soft, like brass, from my dealings with the stuff.
Hack saw?? If it is wearing dremel blades is must be tough.
Ti metal is called a Valve metal. Valve metals will immediately stop coducting if placed into an (say Chloride) electrolyte as an Anode because a very
very tough uniform coating of their Oxide forms that is thick enough to stop the current from flowing (or will get thicker). It is refered to as 'self
protecting under anodic conditions' (call it self sealing or self healing if you like). Other Valve metals are Hf, Ta, Nb, Zr (I think), W and Al
(Aluminium would have the weakest Oxide coat).
If you put enough Voltage to the Valve metal that you have as an Anode then the Oxide coat will break down and current will flow and the Valve metal
will corrode. For Ti I think you need about 20 volts. For Ta you need about 50 volts. Al, a tens of Mili Volts. (These figures may not be exact).
The MMO coating is of course conducting. When a bit of it is scratched off the exposed Ti just anodically protects itself and does not corrode unlike
non Valve metals.See
here for some Ti Oxide info.
Still think someone shoud do a one minute test on a piece of the stuff to see if it is MMO coated before cells etc are put together.
Dann2
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
well I am still working on sealing every little hole in my cell with silicone, I am done with corroded wires...
my klc is on its way...I left it in a car and forgot to grab it, so when ever she stopps by my house i can get it.
ahh i see, that really interesting about that Valve metal. I am guessing that where ANODEizing aluminum gets its name.(and i doubled checked wikipedia
to make sure my guess was right) LOL
next couple days and i should be able to run a test
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
OK OK, I'll weld a shank on one and let rip! A small-scale test with potassium salts won't take long, and if starting with 50 or 60 degree saturated
KCl, and then keeping it at that temp so no KCl falls out, it should make visible chlorate in just a day or two at 40 to 60 amps. It will be a very
harsh test that will check the integrity of the MMO, at a high current density.
Edit - add: Hmm, I'm not so sure anymore. I've probably spot-welded 20+ cathodes and anodes before today with this rig. Normally I don't scrape or
sand the MMO material off first at the contact points, but with this stuff...
The first weld was made; I removed the clamp arms, and CLANG both mesh and shank fall to the floor, quite separate. ZERO adhesion. I tried again,
same result. By dremeling off the MMO at the weld juncture, I was able to weld it up, apparently OK. This behaviour is very much different from my
proven MMO, which welds like crazy, easy as pie, no scraping needed.
There may be some sort of chemical film ON TOP OF the supposed MMO coating. One way or another, we'll find out.
In the end, I think it welded successfully.
[Edited on 8-2-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
Did the current stop flowing yet. (Not being impatent or anything!!!!!!)
If the current flows for a few minutes in a cell without the Voltage rising then I would be willing to bet that is is MMO.
There are very few coatings that would be on Ti that would allow Anodic (ie. +) Current to flow for a few minutes (or less even) without the Voltage
rising (coating being eroded off and Ti Oxide forming) and the current flow stopping.
.
.
.
Has the current stoped yet p;
Dann2
I just heard a statement from the telly by Amelda Marcos.
"They did not find any skeletons in my cupboards, only lots and lots of lovely shoes".
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Well let me toss it into a beaker full of KCl and see what the currents and the voltages look like... hang on.
Edit: 1 liter relatively weak KCl solution - no "used" liquor. Anode dimensions: 2.5" X 2.5". Cathode dimensions, slightly smaller. Spacing,
approx 3/4" or maybe 15 mm.
There is of course massive bubbling and a distinct smell of bleach; a faint smell of chlorine. I will leave it at 4.88V and watch the current, and
will reduce voltage only if heating becomes a problem.
I set the supply to constant voltage, and dialing up from 0, here is what I saw...
V------A
0.00--0
1.00--0
2.00--0
2.72--0.10
3.00--0.60
3.30--1.70
3.50--2.50
3.80--3.80
4.88--10.00
I decided to leave it at 4.88V. It started at 10 amps, and over 1/2 hour, it went to 12.1A. Over 1 hour, to 13.6. The amperage is INCREASING at a
constant voltage.
I switched the supply to constant current; 13.6A and 4.88V. If the trend of increasing conductivity continues, we should see the voltage DROP.
Heating moderate. I have a fan blowing over it and guess the temp to be no more than 50C right now. It is in an HDPE bucket plus a loose-fitting
lid. At 13.6A, the smell of chlorine has increased.
So far, it appears to be functioning the same as I would expect any other chlorate-capable MMO. I see no problems... yet. 
[Edited on 8-2-2009 by Swede]
|
|
|
| Pages:
1
..
19
20
21
22
23
..
48 |