| Pages:
1
..
19
20
21
22 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@agari: using anything already nitrated as raw material is against the competition's rules as well as spirit.
Wake up and smell aga's fart.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by j_sum1  | Ok. But it still does not qualify as a novel route. Convenient? yes. Novel? no.
Prohibited within the terms of the competition? you'll need to ask aga that. But I would read into his above comment that something a bit more
adventurous is required. After all, it is a hefty prize pool and I think you have to earn it. |
Updated the procedure, I didn't "refine" it yet,but the chemicals it calls for should be readily available.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by blogfast25  | @agari: using anything already nitrated as raw material is against the competition's rules as well as spirit.
Wake up and smell aga's fart.
|
My procedure actually specifies how to make the nitrate salt.
Sidenote:Is it just me or is Aga's promise of over 309 American dollars very fishy and we are being trolled?
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
It looks like you copied most of your ideas (and the copper sulfate picture) from NurdRage. Have you actually tried any of this? I'm sure original
photos, perhaps even videos, will be required to claim the prize.
[Edited on 31-10-2015 by MolecularWorld]
As for the amount of the prize:
[Edited on 31-10-2015 by MolecularWorld]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Agari  |
My procedure actually specifies how to make the nitrate salt.
Sidenote:Is it just me or is Aga's promise of over 309 American dollars very fishy and we are being trolled? |
Are you just obtuse or is it you who that's trolling?
Quote: Originally posted by Agari  | Materials:
1 Instand cold pack (Either 86g of calcium ammonium nitrate or just 70g of ammonium nitrate), but not urea.
75g of potassium chloride bought at a grocery store.
|
Shop bought CAN. That's a NITRATE, idiot.
So is nitrate found in composted materials.
The money's real but you're unlikely to get a dime of it. Or start documenting your procedure as required in the opening post.
[Edited on 31-10-2015 by blogfast25]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Okay, time for another potential hypothetical idea open for anyone to try...
Earlier jsum1 tried the destructive oxidation of urea using calcium hypochlorite and lime. It seems to fail because of the formation
of explosive nitrogen chlorides, but it did succeed at destructively oxidising urea, so I am thinking of a non-chloride version.
One version is to scrounge out some manganese dioxide from a couple of new torch cells, filter and wash the dark powder and dry it. Then mix it with
whatever the most concentrated sulfuric acid you can get and add urea. Then attach a condenser and distill off [hopefully] nitric acid and be left
with manganese sulfate hydrate in the flask which can be recycled back as ingredients using electrolysis and carbon electrodes.
The hypothetical reaction is:
8MnO2(s) + (NH2)2CO+ 8H2SO4(l) + heat => 2HNO3(g) + CO2(g) + 8[Mn(SO4).(1.125)H2O](s)
WARNING: This is a novel untried hypothetical reaction! It should be trialed in very small amounts initially to ascertain if it's safe to carry out!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Now that's much more like it !
How clean/concentrated would the H2SO4 need to be ?
Would used battery acid work ?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Purity isn't too much of an issue as you're expected to distill off the nitric acid as it forms, so if the sulfuric acid is too weak, the first
portion of the distillate may be mostly water and so this would be discarded until the good stuff comes over. Long story short, battery acid should be
fine 
I've done a reaction between urea and two equivalents of 40wt% sulfuric acid that after much boiling down results in a sudden vigorous effervescence
(CO2) that forms a crystalline product upon cooling to RT. I hypothesised this to be the double acid salt (NH4)HSO4.[(NH2)3C]HSO4.2H2SO4, but that
reaction only kicks in at quite high temperature and when most of the water has been driven off, so I expect the urea to be oxidised by the MnO2 much
before that (hopefully).
When I first thought up this idea, I was thinking of reacting *that* acid salt with MnO2, but then thought that it would be simpler to do it with urea
straight. However, that is a variation that might be tried, especially if you want to pre-concentrate dilute sulfuric acid.
Anyway, the urea version should do something, we can refine it further after we know what happens.
[Edited on 31-10-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
BTW, the nice thing about MnO2 is that it's a known catalyst for ammonia oxidation (as per the article I attached a long time ago on this thread), so
it seems like a good oxidant to use in a wet chemical approach. Strong sulfuric acid would greatly increases its oxidation strength so that I would
reasonably expect NOx or HNO3 to distill off at high temperature.
Furthermore, the acidic conditions ensure that ammonium nitrate is not formed, but nitric acid. Also manganese sulfate is very hygroscopic, so it
ought to mop up some of the water formed by the reaction.
[Edited on 31-10-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Interesting. I like the idea of urea as a source as it is so easy to get. H2SO4, not so easy round here. HCl and oxalic are readily available though.
Not sure that helps. Still, if we are after a new route, it is food for thought.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The African Process
Just remember, you only need to get a little the first time, because you can easily electrolyse manganese(II) sulfate solutions back to manganese
dioxide and sulfuric acid!!!
In a recycling process, the overall ingredients are just urea, heat, water and electricity making carbon dioxide, hydrogen and nitric acid.
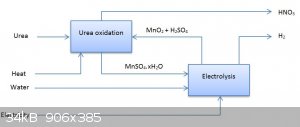
[Edited on 31-10-2015 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Genius.
I lovr the way the carbon violates the law of conservation of mass. 
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Oops, forgot to include that  I'll correct the graphic thanks! I'll correct the graphic thanks!
... now with fewer carbon credits   
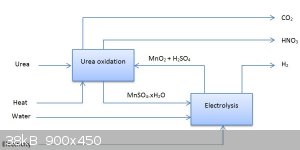
[Edited on 31-10-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Pourbaix diagrams... again!
Okay, thought I'd check out some pourbaix diagrams to see if this is feasible.
MnO2 at a pH of -1 (10M H+ conc.) has an oxidation potential of about +1.3V.
For nitric acid extrapolated at the same pH, it's around +1.2V, so it ought to proceed with strong heating, just-just.
Manganese pourbaix:
http://www.sciencemadness.org/talk/files.php?pid=425582&...
Nitrogen pourbaix:
https://upload.wikimedia.org/wikipedia/commons/c/c9/Pourbaix...
[Edited on 31-10-2015 by deltaH]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by Agari  |
Sidenote:Is it just me or is Aga's promise of over 309 American dollars very fishy and we are being trolled? |
He's good for EVERY bit of it, trust me I know from personal experience his generosity. And in case ya didna know AMMONIUM NITRATE IS a nitrate
fertlizer. Not just for shake and bake. Every slice of bread you eat depends on it.
*edit* misspelled ammonium
[Edited on 10-31-2015 by arkoma]
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's $550 US at today's exchange rates, and yes, it's Real.
Forget any notion of 'fair' or 'organised' - it is what it says in the title - a Drunken Challenge, so the Rules are not enforceable, and could
change.
Basically i'll pay out if someone comes up with something more interesting than just
"Hey ! New Idea ! Distill a Nitrate Salt with Concentrated Sulphuric acid !"
Someone better come up with something soon or i'll have to post the process the lizard aliens use and win it myself so we can get on with DAC#7 -
AntiGravity.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by aga  | It's $550 US at today's exchange rates, and yes, it's Real.
Forget any notion of 'fair' or 'organised' - it is what it says in the title - a Drunken Challenge, so the Rules are not enforceable, and could
change.
Basically i'll pay out if someone comes up with something more interesting than just
"Hey ! New Idea ! Distill a Nitrate Salt with Concentrated Sulphuric acid !"
Someone better come up with something soon or i'll have to post the process the lizard aliens use and win it myself so we can get on with DAC#7 -
AntiGravity. |
Will nitrates found/produced in compost violate the rules?
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
I realize its 11 pages long, but why don'tcha READ the THREAD?
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
@arkoma : I see 21 pages. Still readable within a reasonable amount of time.
I can't speak for aga, but I'd imagine you'd have to start with fresh compostables or manure (or a non-nitrate compound extracted from fresh
compost/manure), and provide a complete procedure (with photos!) for turning it into and extracting nitrates; then provide a complete procedure for
turning the nitrate into nitric acid using only common household items (with more evidence you actually tried it), to have a shot at the prize.
[Edited on 31-10-2015 by MolecularWorld]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Something like that.
A quick google, cut-n-paste to this thread will not win anything.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK.
I'm getting bored with this whole thing.
How hard is it to break out of the 'distill a nitrate with conc sulphuric acid' mindset ?
So many times that has been suggested, clearly by people with zero understanding (or reading abilities).
Recently deltaH broke the 'dissolve in Nitric' mould for Gold dissolution, using salt, vinegar and bleach.
Perhaps the prize fund for this DAC was too small to attract serious interest.
If no actual useful and qualifying process is posted within 6 months i'll post my own submission, win, and be able to move on to something else much
more exciting.
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
6 months? Mine should be ready before then. And it shouldn't require input of nitrates or sulfuric acid.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cool !
I look forward to seeing your process.
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
No details yet; I'm not yet certain that it'll work, and I don't want anyone to get a jump on me if it does. I will say that I found everything I
[think I] need at a single Walmart, including materials -and- apparatus (mason jars... LOTS of mason jars!). Whether it works or not, I should have
something useful posted before the New Year.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sounds Good !
We can call it the Single-Wallmart MolecularWorld process - if it works ...
|
|
|
| Pages:
1
..
19
20
21
22 |