| Pages:
1
..
18
19
20
21
22
..
27 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Urea is a very weak base and so Urea Nitrate (UN) is mediumly acidic while Ammonia is a stronger base what makes Ammonium Nitrate (AN) less acidic.
Medium acid is no good for NG because UN hydrolyses back to HNO3 and NH2-CO-NH2 quite easily.
This will ruin the basic wash devoted to stabilise the NG!
Good advice...don't ever store your UN/NG or UN/EGDN dynamite or whatever nitric ester with UN.
You might get spontaneous runnaway, fire or detonation of your batch...just as uncorrectly washed nitrate ester would do.
[Edited on 9-8-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Ral123  | | UN has very low energy but I've heard it can be set off with 2g HMTD. Also I'm not sure if it's easy to tell when the UN is completely dry, but still
hasn't lost HNO3. I believe UN+NG/EGDN can make cheap dynamite with decent brisance and density(may be densities that are too high for ammonium
dynamite). |
Actually urea nitrate does not have very low energy but has good energy, and no it can't be set off with 2 grams of HMTD, and it is very easy to tell
when it is dry, and it is stable and won't lose HNO3 at ordinary temperatures. There is a simplicity and economy that is an advantage for using urea
nitrate alone. The difficulty encountered is that it is only barely cap sensitive to a very powerful detonator, however once it is detonated high
order it works just fine.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Half a kilo charge on the surface barely made a crater to fit a shoe. Although it was heavily beaten with a ceramic rod and felt quite dense, it went
off with 15g AP. You could find pieces of the plastic jar in the smoking crater. Yes somewhat dry ,pressed UN can be a cap test may be, but I don't
see it as an accurate one.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Ral123  | | Half a kilo charge on the surface barely made a crater to fit a shoe. Although it was heavily beaten with a ceramic rod and felt quite dense, it went
off with 15g AP. You could find pieces of the plastic jar in the smoking crater. Yes somewhat dry ,pressed UN can be a cap test may be, but I don't
see it as an accurate one. |
I won't argue with your tests, methods, or opinions. There is plenty of good information published already. Used alone or in OB compositions, this
material has proven to be a quite good mining explosive having plenty of velocity and heaving energy for the purpose, regardless of your tests or
opinions to the contrary.
Some urea nitrate / ammonium nitrate / urea compositions are nearly as brisant as TNT and have greater total energy, but can be difficult to detonate,
and 15 grams of AP does not a special engineers #16 detonator make.
[Edited on 10-8-2014 by Rosco Bodine]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I don't give UN more then R.E. 0.8, specific energy less then ANFO and whopping 4700m/s velocity. Once BP was a good mining explosive also.
TNT is something completely different. For example it can be used for a shaped charge.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by deadpool  | @ Henning Brand. First good job on your success; however, I have serious safety and lab concerns with your coffee pot! The top of the “pot”
looks to be plastic, and I’m quite certain this would react to the nitro/sulfuric acids making a mess (how did you even get the acids out?). The
picture of your mix acid/glycerin solution almost appears the same color as the plastic, which makes me wonder if this was due to plastic
contamination or nitrogen dioxide in the nitric acid. Also please use a sealable container and not plastic wrap! The plastics wrap can react to
nitric acid, not to mention just being in proximity to nitroglycerine can cause serious headaches, and a drop in blood pressure. Safety note worth
mentioning: Do not use latex gloves they combust when in contact with nitric acid (seriously violent awesome to see), nitrile gloves are poor
protection, vinyl is best. Here are some pointers to follow that might help, if you used a fuming acid solution, this will increase your yield
because it will facilitate water as a leaving group from the glycerin during nitration. Have you considered nitrocellulose as binder? Keep in mind,
this will be a high detonation velocity depending of the nitrogen content of the nitrocellulose. In cold climates, substitute the glycerin for ethyl
glycol, end product has much lower freezing point.
[Edited on 8-8-2014 by deadpool] |
No need to worry about me, I have been making that stuff for probably about a decade now; dozens of times.  I have considered everything you have mentioned many times. The coffee pot does have an non-ideal material for its
top, but it had a very nice shape for swirling and was about the right size for the job. The same type of coffee pot is available with a glass spout
and handle attached on the outside with a steel band, but the one with the plastic spout in my possession came from a thrift shop for $1-2 dollars.
The orange/red color comes from the dye, or whatever was in the commercial drain cleaner I used as the source of sulfuric acid. The acid was simply
poured out of the coffee pot via the pour spout. I noticed slight discoloration in the plastic spout but nothing serious. You are right that more
concentrated acids, with less water, would result in a higher yield of NG. I decided to use the lower concentrated acid anyway because it would
produce enough for my purposes and the gain in yield didn't seem worth the extra time and energy required to re-distill the nitric acid and/or
concentrate the sulfuric acid further. I have found plastic wrap and polyethylene bags to be suitable for covering the tops of vessels containing
sulfuric and nitric acids (at least temporarily). Fuming nitric acid fumes degrade the surface of the plastic over time, but it never seemed to be a
cause for serious concern. I have considered everything you have mentioned many times. The coffee pot does have an non-ideal material for its
top, but it had a very nice shape for swirling and was about the right size for the job. The same type of coffee pot is available with a glass spout
and handle attached on the outside with a steel band, but the one with the plastic spout in my possession came from a thrift shop for $1-2 dollars.
The orange/red color comes from the dye, or whatever was in the commercial drain cleaner I used as the source of sulfuric acid. The acid was simply
poured out of the coffee pot via the pour spout. I noticed slight discoloration in the plastic spout but nothing serious. You are right that more
concentrated acids, with less water, would result in a higher yield of NG. I decided to use the lower concentrated acid anyway because it would
produce enough for my purposes and the gain in yield didn't seem worth the extra time and energy required to re-distill the nitric acid and/or
concentrate the sulfuric acid further. I have found plastic wrap and polyethylene bags to be suitable for covering the tops of vessels containing
sulfuric and nitric acids (at least temporarily). Fuming nitric acid fumes degrade the surface of the plastic over time, but it never seemed to be a
cause for serious concern.
Especially when done on a small scale, a NG synthesis does not require the same precision as analytical work does. Often times, especially in small
scale syntheses, saved time is more valuable to the synthetic chemist than a higher yield.
Regarding urea nitrate, has it ever been used commercially? I know it has decent power and is cost effective, but I thought it had stability and/or
compatibility issues that kept it from being used as a commercial explosive.
[Edited on 11-8-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Ammonium nitrate has higher density than urea nitrate and than nitroglycerin.
So overal density of the mix AN/NG will be higher.
But AN and NG both display a positive OB...so maybe would it be good to add some high density fuel to increase power.
UN/NG mix would have a lower density than AN/NG mix. But UN has slightly negative OB what means there exist a Perfect OB mix between NG and UN to
reach optimum energy release.
One step further would be the use of nitroguanidine/NG or nitrourea/NG perfect OB mix 
Or even better replacing NG by ETN, XPN, MHN in the mixes to reach better performances!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Vikascoder
Hazard to Others
  
Posts: 309
Registered: 28-1-2012
Member Is Offline
Mood: No Mood
|
|
Making urea nitrate is very cheap but for detonating it u will need a powerful detonator .
Recently I made a charge of 200gm urea nitrate mixed with charcoal and packed in steel pipe of length 6 inch and diameter 1 inch detonated by2 gm rdx
and 1 gm mercury fulminate detonator . After detonation it made fair noise and nothing happened to ground but no pieces of steel pipe were seen don't
know where they had gone
Girls break promises like a small child breaks pencil tips so don't trust girl bcoz no girl=no tension
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Hennig Brand  | | Regarding urea nitrate, has it ever been used commercially? I know it has decent power and is cost effective, but I thought it had stability and/or
compatibility issues that kept it from being used as a commercial explosive. |
Yes I believe it has been used commercially and is mentioned at least as a component in some patent compositions. The PATR article mentions that an OB
mixture with KNO3 is regarded as one of the best blasting explosives. I'm sure if money is no object anything could be used for blasting, but where
the economics are governing, the cheapest useful explosives for the job will be the winning bid for the contract.
Using a double salt of urea and sodium nitrate or a double salt of urea and calcium nitrate might provide an energetic fuel value that is needed for
OB in a mixture as is proposed by franklyn, as an alternative to the urea peroxide.
http://www.sciencemadness.org/talk/viewthread.php?tid=16785&...
I think it likely any of these type OB mixtures are going to have higher performance than ANFO, smaller critical diameter, and higher velocity and
brisance, and will be more sensitive. These compositions are sort of intermediate between ANFO and NG dynamites......but are cheaper than NG
dynamites, and are similar to tovex type water gels or emulsions in power, but even cheaper.
There are some related posts that are worth considering
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=15019&...
I'm sure there is much more.
I recall Philou Zrealone describing OB mixtures of urea nitrate and ammonium nitrate as potentially useful, and there are ways of incorporating these
materials in a foamed melt cast that is cap sensitive and may be more sensitive than granular urea nitrate used alone. But even just plain dry
crystalline urea nitrate when detonated completely by a powerful detonator should have plenty of power sufficient for ordinary blasting uses.
[Edited on 14-8-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Interesting, thanks for the links. I have tried some of the ammonium nitrate/urea nitrate mixtures. With the mixtures I made the two components were
simply dry mixed. I found a few pictures from a couple of months back where about 1kg of urea nitrate was put in an empty aluminum oxygen tank for a
small portable cutting/brazing torch and used on a stump. The aluminum tank was about the same size as a handheld propane plumbing torch tank. The UN
was simply funnelled into the cylinder, and the cylinder was tapped on the table every so often to settle the charge. For initiation, a well pressed
3g ETN base charge was used. It looked like a complete detonation, but the power was extremely low in comparison to even the lowest velocity dynamite.
At least the stump will be fairly easy to remove now, since the earth was cleared out from around the roots and the tap root was sheared off.
When I try urea nitrate again I will use a considerably more powerful initiating charge and see if that makes a difference. Attached are three of the
post detonation smoke scenes just because they look nice.
        
[Edited on 14-8-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
If you used a 3 gram ETN detonator then that is more than enough for urea nitrate. I don't know what is the issue that would cause low output. Even a
Kg of black powder should have done the job there. PATR gives a ballistic mortar test value for urea nitrate at 92% of TNT .....so something wierd is
occurring with these anomalously low outputs being reported. From the charge you used, I would have expected the stump to be tossed in pieces high
into the air and nothing left but a smoking hole where it used to be.
Attachment: Urea pages from PATR Vol. 10.pdf (1.2MB)
This file has been downloaded 854 times
[Edited on 14-8-2014 by Rosco Bodine]
Attachment: FR585671A Urea Nitrate mixture for blasting.pdf (63kB)
This file has been downloaded 1046 times
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The urea nitrate may have had questionable purity, but I have had similar results other times as well. Without supplemental heating, it can be a long
& tedious process to get urea nitrate really dry. If the heating is not done very carefully, decomposition can easily occur. I used one of those
glass heating trays for keeping food warm at the table. A large clear glass casserole dish was set on top of the heating tray and is what held the UN
to be dried. The heating tray is rated at 250 watts, which I found is more than enough to decompose the UN when used the way it was used. I was
careful to discard the material that had obviously decomposed, but the charge was likely still of reduced purity as the result of contamination
produced from decomposition. The density of these charges is also very low, but you are right, they should still function better than this.
How much decomposition and reduction in purity would it take to significantly drop the sensitivity of the charge? I am guessing not very much. How
much moisture would it take to significantly drop the sensitivity? I am also guessing not that much. I did have that one positive test years ago, with
what seemed like good performance, that I mentioned earlier. I remember air drying the UN with no supplemental heating for several days and using 2-3g
of well pressed PETN to initiate the charge in a steel propane cylinder. The only part of the synthesis that I believe I could have screwing up was
the drying of the product. I wick away as much moisture as possible before drying, with large cotton towels, but even so drying is a long and tedious
process. I have seen old posts by Philou at the old E&W forum where a final wash with a more volatile solvent than water was used before drying.
Sounds like an option but it would increase cost.
I have since put a light dimmer on the glass heating tray, so that the heat can be turned down. I imagine this should solve the excessive
decomposition problem. Urea nitrate is definitely not thermally stable and must be dried carefully. I may give it another try.
[Edited on 14-8-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I did not experience any "long and tedious" drying issue with urea nitrate. It isn't hygroscopic and dries easily to a mild cake of crystals but
easily broken apart lumps that results in free flowing loose glittering crystals, about like table sugar. When filtering about a kilo of the
material, I filter it through a cloth from an old pillow case or dress shirt draped in a one gallon funnel, and when it finishes gravity draining,
gather the edges of the cloth and twist tightly the grapefruit sized ball of crystals to squeeze out as much liquid as possible, and then place the
still tightly twisted ball onto a folded several thicknesses bath towel to wick out as much residual liquid as possible. Maybe let it sit there for an
hour on the
blotter and the crystals are almost dry already, then break up the ball of crystals in a large glass oven tray . I heated my tray by using a cheap
heating pad like is used for sore muscles, laid on a half inch thick stack of newspapers on a sheet of plywood. Set a fan to move fresh air across the
crystals and they are bone dry in a few hours. Break up the cake of crystals with a a potato masher and they fall apart easily and completely to the
point you don't have to screen them, but just dump the tray into a container. So far as comparing physical properties at mild temperatures with
another common crystal, urea nitrate is a lot like potasium nitrate.
[Edited on 15-8-2014 by Rosco Bodine]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
The reason why the charge of UN made by Henning did not yielded the expected results may lie in the density of the charge itself. The charge is
essentially made of loose granule of UN so it's density is fairly low. Low density+low energy explosive (UN) make a low performance explosive charge.
Another reason may lie in a partial detonation of the charge. Partial detonation were unreacted explosive can be found is seen in ammonium
nitrate-salts explosive, quote from [1], pp. 66:
"In a classic paper, Miron, Watson and Hay, presented experimental evidence that large amounts of the ammonium salts remain in the explosive
residue left from exploded ammonium salt-explosive mixtures used as commercial explosives in a 3.7 meter diameter steel sphere with the inside surface
metallized with aluminum. Up to 50% of the ammonium nitrate was recovered from the explosion residues of granular, water-gel, and gelatinous
explosives while no oil was recovered. Large percentages of sodium chloride, sodium nitrate, calcium carbonate and even nitrocellulose were recovered
from some of the commercial explosives. Up to 96% of the aluminum was recovered in the residue of an aluminum containing water-gel explosive."
Reference:
[1] Charles L. Mader, Numerical Modeling of Explosives and Propellants, Third Edition, 2008.
Dany.
[Edited on 15-8-2014 by Dany]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That paper seems like a commentary about inadequately boosted insensitive charges showing partial detonation.
When complete detonation occurs, residues are generally only detonation reaction products and there are not even trace residues of undetonated
material. Many explosives including picric acid for example don't even leave microscopic traces of undetonated material, and with a dye it is easy
enough to see in the absence of color in the test chamber washdown water.
It might seem like it would be the low density and that might cause some energy reduction but not the extent of reduction that occurred. The energy
of UN is good even at a loosely poured bulk density which is the same or similar uncompressed charge as would generally be used in a bore hole into
which is simply poured for example loose charges of prilled ANFO. There is some drop in velocity but most of the total bubble energy of the charge
weight is still realized and for a heaving earth type of work the reduced velocity is actually a benefit. For something like stump blasting it is
wanted to eject the stump from its socket in the earth more than it would be desired to shatter the stump to pieces.
[Edited on 15-8-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I think lately I have been doing basically everything you suggested above in terms of drying procedure, but probably not years ago when it took
several days to dry the UN. The only significant difference this time, from your procedure, was that I used a more powerful heat source which resulted
in some decomposition of the UN next to the glass dish. In my limited experience UN will gladly go low order if given a choice and for high output it
needs to be overdriven. There are many factors which could affect the sensitivity of a UN charge to initiation and the minimum initiator requirement
to achieve high output.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There is a possibility that ETN used as a base charge could have gone low order and that may have coupled with a low order detonation of the UN. I
have only used picric acid base charge detonators, and PA does not low order detonate in my experience if it completely detonates it always goes high
order. It could also occur that a PA base has a catalytic effect towards coupling with the UN and causing it to high order also. I'm not sure what
is going on with your low order and low output from UN, but I'm sure that differs from my own observation of UN.
Looking at the link where Bert showed tannerite in a small column diameter in PVC loosely loaded .....it is easy to see there is still plenty of
energy developed by a loose charge....so don't make too much of charge density versus blast effect except for the more local direct contact or jet
effect of close contact of cutting charges. Those are more of a microclimate localized "point contact" effect for a charge than is the larger "bubble
energy" kind of blast effect as comes into play for blasting charges.
[Edited on 15-8-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I am inclined to believe most, if not all, explosives can detonate with a low output if not properly initiated. From old explosives texts and my own
experiments, I know picric acid can definitely have diminished output if not driven hard enough. I always thought that ETN either went high order or
not at all, but I did a couple of tests with ETN plastique in the last while which showed that these types of explosives can detonate with very
different velocities depending on how they are initiated (at least in small charge size). Larger charges may self accelerate to the higher velocity, I
don't know.
I think the problem me and many others are having with urea nitrate is thermal decomposition during the drying process. I found an interesting article
which shows that urea nitrate can be easily thermally decomposed, especially in the presence of acidity. I imagine my urea nitrate was heavily
contaminated with decomposition products.
Attachment: Decompositions of Urea and Guanidine Nitrates.pdf (211kB)
This file has been downloaded 1454 times
Here is an interesting table from the appendix of the report.
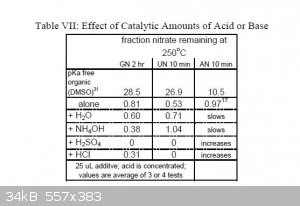
I would have liked to have seen results for lower temperatures.
[Edited on 16-8-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
1 kg of UN should have tossed that stump the same distance as a pair of 1lb blocks of TNT. Why that did not happen I'm not really sure. You already
know how difficult it is to get picric acid to detonate fully and it is a lot worse for trying to detonate TNT which is even harder to
detonate......and urea nitrate has about the same sensitivity as TNT. So it could be that it isn't being overdriven enough to get it to go. You don't
want the UN to be powdered but just plain crystals loosely poured and only gently settled, as the more densely it is packed the less sensitive it will
become, and it is already difficult enough to detonate. However, so is TNT difficult to detonate, and a lot of other relatively insensitive
explosives, which once they do get going have good energy.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I appreciate your insight. I am going to give UN another try in the next while. I really think it has been my impatience with drying that has been the
biggest problem, but I will need to test that out to know for sure.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here is a video of a UN loose charge in an open plastic bowl reportedly 700 g UN
as an example ......not my video but this would appear to be about right. I'm not sure what is the development concept of the particular booster mix
and detonator, and it may have just been a trial and error kind of approach. I think a hot enough detonator should give similar results in a simpler
configuration. A loose charge is as simple as it gets, pour it in a ziplock bag or an empty plastic pop bottle and shoot it. From the video you can
see the blast effect for a loose charge is considerable, given the time delay for the falling debris, one piece of the tire was blown est. 500 feet or
more straight up into the air. That same charge in the earth underneath a stump should I think have been plenty to do the job of convincing that stump
to jump out of the ground. So a full 1 kg like you were using with confinement of an aluminum cylinder would seem to have been plenty.
https://www.youtube.com/watch?v=5SpTofaUY0I
<iframe sandbox width="640" height="360" src="//www.youtube.com/embed/5SpTofaUY0I?rel=0" frameborder="0" allowfullscreen></iframe>
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
That looks more like it. I am going to use a more powerful detonator/booster the next time I try it. I get so used to initiating dynamites, which can
be initiated with the weakest caps, that it clouds judgment when it comes to these more insensitive materials I think. I need to learn to be more
generous. 
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Mud capping the charge, or wetting and packing the earth in the bore hole after charge placement could also help if the blast is escaping through the
loose dirt and force is being lost mostly blowing around the target instead of against it. A fence post hole dirt auger would be handy for boring
under a stump. Set the charge and then dump a bucket of mud atop the charge in the bore hole to mud cap it before firing.
|
|
|
z13123
Harmless

Posts: 4
Registered: 20-9-2014
Member Is Offline
Mood: No Mood
|
|
First time making NG I used:
2mL glycerin
8mL HNO3 distilled from KNO3 and H2SO4
12mL 98% H2SO4
washed many times with water, then baking soda, rinsed in NaCl solution after it was neutral, rinsed again with water, extracted, and did a flame
test, it took a while for the water to boil out (I didn't dehydrate it) but it burned very very quickly with a poof and a blue flame appeared. I
burned the rest of this solution because I felt it wasn't worth the effort to dehydrate it. I yielded around 3mL of NG.
Next, I went larger scale, with the same proportions and 25mL of glycerine. I yeilded around 40-45mL before dehydration. I have not yet finished
dehydrating it. It was very cloudy and milky looking, but after sitting in a box with silica gel on the bottom of it for half a day, it looks clear,
but with a slight yellow tinge still.
I was reading through the pages in this discussion and someone mentioned not using a stirring rod. I was using a glass stirring rod and was wondering
if this was an issue and if it could detonate the NG while washing/mixing. Also, because I don't have a separation funnel (I know... I should get
one), I used a syringe to suck out the NG, and squirted it out into water to wash it. I noticed while doing this, some of the NG (looked oily-like)
floated to the top. Any ideas on how to prevent this/what it is? Also, any ideas on the best temperature to store it? Should I freeze it in my
fridge or just keep in a room temperature place? Also, thoughts on using a black powder charge to detonate it? I don't have AP, PETN or any common
blasting caps available.
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Um, I probably shouldn't spoon feed you here but this is pretty dangerous stuff here so ill help with an answer other than utfse.....Why did you make
it without a means to detonate it?
Glass should be avoided as a hard clink on the beaker could be catastrophic. With 25mls it would likely kill you. Black powder was what they ward in
the old days but it works, not well but it does, and you won't be making the most out of your NG.
since you made the nitric acid you should be able to devise a way to a primary. Use it up and avoid storing it as it doesn't keep well. Did you test
with PH paper? It MUST be neutral or it is incredibly dangerous to store. There is stacks of info on this stuff all over the internet. It would be
wise to research it some more before something bad happens. NP
|
|
|
| Pages:
1
..
18
19
20
21
22
..
27 |