| Pages:
1
..
18
19
20
21
22
..
33 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's cos they do not absorb/emit any photons in the 390nm to 700nm range, having carelessly donated their sole 4d electron so some charity case or
other.
Some elements are dangers to themselves.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
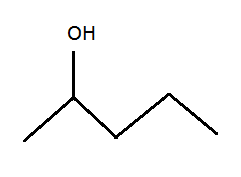
Draw all isomers of this alcohol:
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
In MS Paint ?
You're kidding.
Numbering the carbons left to right, the OH can go on the 2, 3 or 4 postions.
Not entirely sure why it can't go on 1 or 5, but it just feels like it can't.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | It's cos they do not absorb/emit any photons in the 390nm to 700nm range, having carelessly donated their sole 4d electron so some charity case or
other.
Some elements are dangers to themselves. |
Correct. No d-electrons, no transitions. 10 (full set!) d electrons, no transmissions either... simples really! So 0 and 10: no colours.
Important corollary:
f block elements are also often colourful for similar reasons.
The 4 d orbitals also split into two groups according to orientation and thus slightly different energy levels, in a ligand field. An incompletely
filled 4d orbital thus also gives rise to transitions with emissions in the VIS part of the electromagnetic spectrum. Most (+3) lanthanides show
colour, as do many actinides due to part-filled 5d orbitals.
[Edited on 22-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | In MS Paint ?
You're kidding.
Numbering the carbons left to right, the OH can go on the 2, 3 or 4 postions.
Not entirely sure why it can't go on 1 or 5, but it just feels like it can't. |
It can be done in MS Paint-in-the arse because the shapes are simple.
It can go from 1 to 5: it's a pentanol. No naming was requested (Darkstar will do the IUPAC incantations later on, I think).
[Edited on 21-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
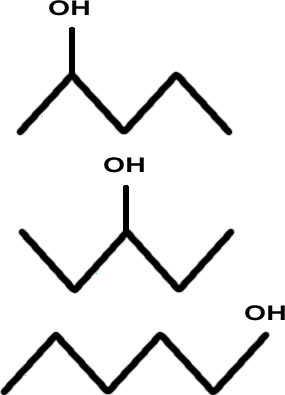
'owzat ?
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
There are other isomers. Keep on trying.
(And some of the compounds have two enantiomers, but I'm not sure we're counting those.)
[Edited on 11-22-2015 by Cheddite Cheese]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Not bad but there's two more.
Think about methylbutane...
Try also the phenol question. Then I'll QC explain strong and weak acids, very briefly...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Cheddite Cheese  | There are other isomers. Keep on trying.
(And some of the compounds have two enantiomers, but I'm not sure we're counting those.)
[Edited on 11-22-2015 by Cheddite Cheese] |
Sorry, CC, the question specified alcohols. Sorry if that wasn't clear.
I wasn't counting enantiomers but point them out, by all means.
[Edited on 22-11-2015 by blogfast25]
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Aren't there three (not two) more alcohols?
[Edited on 11-22-2015 by Cheddite Cheese]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You're correct: there are three more.
And two out of the six are enantiomers, making a total of 8.
[Edited on 22-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
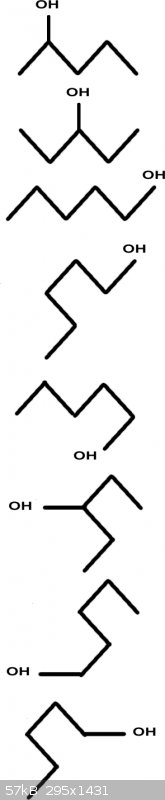
More quiggles
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
4, 5, 6 etc are conformations, not really isomers in the intended sense of the word.
Hint: try starting from 2-methyl butane, add the OH and see what you get. A C5 alcohol does not require all Cs to be 'inline', branching can still
make a 'C5 ol'.
[Edited on 22-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
More squiggles !!! OK.
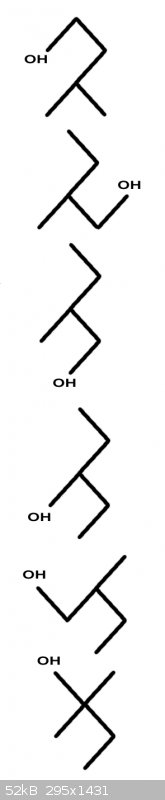
Hmm
Looks like Foothark to me.
[Edited on 22-11-2015 by aga]
[Edited on 22-11-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The first three you started off with were fine and the very first one (pentan-2-ol) is an enantiomer. The others again are conformations.
Here are the other three, with the second one being an enantiomer:
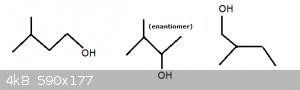
The way to find enantiomers is to look for carbon atoms that are bonded to four different groups.
Take the middle one in my drawing. The C-atom bonded to the OH group is also bonded to a methyl group, an isopropyl group and finally a hydrogen atom.
Four different species.
Such a molecule, when depicted in 3D always has a mirror image molecule. Together the pair are a pair of enantiomers.
[Edited on 23-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The Volume of information in this thread is a little overwhelming for a part-time amateur.
It is amazingly condensed and informative, and well explained, therefore accessible.
In order to understand it more thoroughly (as it deserves) i've decided to cut down on the beer intake and devote the extra brain-function to learning
this material better.
This a dangerous undertaking : the last time i stopped drinking altogether is when i took up Chemistry, so there's no knowing What may happen.
[Edited on 22-11-2015 by aga]
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Neopentanol (aka 2,2-dimethylpropanol) hasn't been mentioned yet. That was the third one I was thinking of.
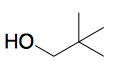
[Edited on 11-22-2015 by Cheddite Cheese]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
More information overload fer aga (all resistance is futile).
Enantiomers are also referred to as optical isomers because the two isomers (sometimes called S and R) change the polarisation orientation of
plane-polarised light in opposite ways. Guess what? That's a QM effect too!
If you can stomach it, here's a decent explanation on how that works.
[Edited on 23-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yup. Good one. Should have looked at propane-based pentanols too.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And while we're still talking about alcohols, don't forget this question about phenol... It's very informative, I promise!
[Edited on 23-11-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A little Ode to Aga...
We've decided not to publish the scores of the QQuiz' participants but it needs to be said that aga's score constituted a Big Fat Pass (albeit perhaps
not 'cum laude') Considering that this imperfect and incomplete course crams in material in about 4 months that at GCSE or A-level would take a full
school year, that score is for a near-complete novice in fact an achievement. His 'dogged pursuit of knowledge' is equally commendable.
So three cheers for aga! The prize (a dented pingpong ball and a 'I heart Suzie Q' sticker manually modified to read 'I heart QM') is
in the post!
Light relief:
I found this nice little page on alkane isomers that lists these isomers up to tetradecane (1858 isomers for that one!) The short introductory
video is also worth watching.
[Edited on 23-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
A Pass ?! Erm, are you absolutely sure ? Astonishing.
The glory is all yours blogfast, not forgetting Darkstar's superb OC summary.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Aga: Ok then. Now let's look at the following (fairly big) chunk.
Express recap of Brønsted–Lowry (BL) acid base theory:
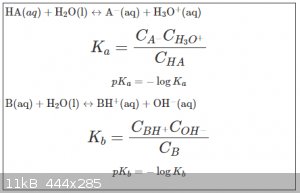
The indexed Cs in the K equations represent actual concentrations (assuming a dilute solution), not what it says on the bottle (for “0.1 M acetic
acid” e.g. the C<sub>HA</sub> is always (slightly) lower than the nominal concentration of 0.1 M).
It has to be noted also that A<sup>-</sup>, the so-called conjugated base of HA, is in fact also a weak base. See the alkaline solutions
of acetates, carbonates, bicarbonates etc.
If B is the conjugated base of HA then we can even derive (derivation not given here) that:
pK<sub>a</sub> + pK<sub>b</sub> = 14
K and pK values thus offer an objective criterion by which we can rank acids and bases from strong to weak.
Stabilisation of Phenoxide anion:
The deprotonation reaction of phenol in water is of course:

We know that non-aromatic alcohols react neither acidly nor basic, so what prompts phenol to be such a (very!) weak acid? The answer lies in the
stabilisation of the phenoxide anion due to de-localisation of the negative charge. The full explanation can be found here. A de-localised negative charge is less attractive to protons.
Why strong acids are strong acids:
Let’s recall the case of HBr:
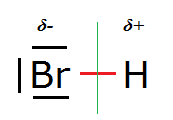
The H-Br bond is highly polarised due to the large difference in electronegativity between Br and H. In the presence of a willing proton receptor like
water the deprotonation reaction of HBr runs to completion (equilibrium fully to the right). HBr solutions contain almost no free, undissociated HBr,
containing only bromide and oxonium ions (and solvent water).
Cross-paradigm agreement:
In a decent country with reasonable laws and rules these regulations apply from north to south and east to west.
The same is true of a good scientific paradigm (set of consistent theories). And here we have quite an interesting case of correspondence between
Brønsted–Lowry acid base theory and its complementary Lewis acid base theory.
Consider the case of an interesting chloro-complex, called hexachlorostannate:
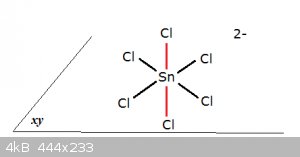
Without going into MO structure (which is simple) the black orbitals lie in the xy-plane, the red ones align with the z-axis. The structure is in
accordance with a typical EX<sub>6</sub> molecule, an octahedron.
Cl<sup>-</sup> can be seen as the conjugated base of HCl, an acid slightly stronger even than HBr. By definition higher up
Cl<sup>-</sup> must be an extremely weak base, as of course evidenced by the neutrality of NaCl solutions.
So if Cl<sup>-</sup> is a weak BL base, is it also a weak Lewis base? Actually, yes.
Firstly, is it a Lewis base at all? Yes. In hexachlorostannate, the bonds are formed by six Cl<sup>-</sup> donating one of its four lone
pair electron to empty hybrids (sp<sup>3</sup>d<sup>2</sup>, to be precise). That’s the essence of a Lewis base: an electron
pair donor.
Hexachlorostannate salts (ammonium and alkali metals) can be crystallised easily from water. But they’re not hugely stable:
1. Adding a little ammonia to their solutions immediately precipitates Sn(OH)4. Adding stronger alkali leads to formation of stannate:
Sn(OH)<sub>6</sub><sup>2-</sup>. This due to ligand exchange: hydroxide ions are a far stronger Lewis base.
2. Heating K2SnCl6 to above the BP of SnCl4 causes the latter to distil off.
Many other chloro-complexes are far less stable: tetrachlorocuprates and tetrachloroferrates exist in solution but cannot be isolated from them (at
least not by simple evaporation of solvent), KAlCl4 does exist but can’t be recrystallised from water. All three are subject to ligand exchange with
hydroxide ions (ammonia solution, for instance).
So Cl<sup>-</sup> is a very weak BL base and isn’t all too great as a Lewis base either.
[Edited on 24-11-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  |
...
Without going into MO structure (which is simple) the black orbitals lie in the xy-plane, the red ones align with the z-axis. The structure is in
accordance with a typical EX<sub>6</sub> molecule.
...
In hexachlorostannate, the bonds are formed by six Cl<sup>-</sup> donating one of its four lone pair electron to empty hybrids
(sp<sup>3</sup>d<sup>2</sup>, to be precise). |
Being a mere 'Pass' retard, the first thing i did was go look at the electron configuration of Sn. It didn't help.
Not having re-read and fully comprehended the whole thing yet, it appears that i missed something fundamental in how the hybridisation happens (maybe
other key bits too).
A step-by-step explanation of what the electrons are doing in the formation of this ion would be very very helpful.
Pretty pretty please.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
A step-by-step explanation of what the electrons are doing in the formation of this ion would be very very helpful.
Pretty pretty please. |
Alright, but only 'cos you ask so nice...
Sn ground state: [Kr] 4d<sup>10</sup>5s<sup>2</sup>5p<sup>2</sup>.
The central Sn ion in the hexachlorostannate ion is in oxidation state +4, so it has lost these four
5s<sup>2</sup>5p<sup>2</sup> valence electrons, these orbitals are now empty.
The empty 5s, three 5p (one p<sub>x</sub>, one p<sub>y</sub> and one p<sub>z</sub> and two 5d orbitals (also empty) now hybridise into 6
sp<sup>3</sup>d<sup>2</sup> orbitals, all empty. and two 5d orbitals (also empty) now hybridise into 6
sp<sup>3</sup>d<sup>2</sup> orbitals, all empty.
The chloride ions are soft Lewis bases because they each have 4 lone electron pairs: electron configuration of Cl<sup>-</sup> is [Ne]
3s<sup>2</sup>3p<sub>x</sub><sup>2</sup>3p<sub>y</sub><sup>2</sup>3p<sub>z</sub&g
t;<sup>2</sup>.
Each chloride ion now donates one of its lone electron pairs into one of the 6 empty sp<sup>3</sup>d<sup>2</sup>. Each
molecular orbital resulting from this is full. These MOs are essentially σ MOs.
Clearish, squire?
[Edited on 23-11-2015 by blogfast25]
|
|
|
| Pages:
1
..
18
19
20
21
22
..
33 |