| Pages:
1
2 |
Dr.Bob
International Hazard
    
Posts: 2750
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Another nice trick is to tape a small test tube to the side of commonly used reagents/solvents that will hold a Pasteur pipette inside it. Then leave
one in there such that it can be reused each time a few drops are needed. This only works for chemicals that evaporate and don't go bad, but it is
great for DCM, TLC solvents, etc that you need a small amount of often.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Paper Towel Trick
When I pour dangerous things, I always do this:
I take a piece of paper towel, and fold it in half.
I then keep folding it in half the same way until I have a 12"x1" strip of paper towel.
I wrap it around the bottle above the label, but below the neck.
Pour the acid.
Check the towel for moisture. If it has acid on it, I wipe the side of the bottle.
It is a good trick. The only problem I can see is if you have trouble holding the bottle and paper towel in the same hand. It might be slippery! If
that is a problem, tape the paper towel where the two ends meet, or put a rubber band around it (so you just have to worry about holding the bottle!).
Hope that helps!
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Twospoons  | Why not keep a pipette specifically for each chemical you need to dispense? No contamination that way.
I always use a glass pipette, with a rubber squeeze bulb for my acid. No choice really - ever tried to pour a few ml from a 10 litre bottle of
nitric?
[Edited on 5-2-2008 by Twospoons] |
i buy all my acids in 5l containers, i just buy 1 litre bottles for a buck and a half each, and pour a litre into that bottle, its a lot easier to
handle, especially with H2SO4 because its so heavy
all above information is intellectual property of Pyro.  |
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Huh, I was very surprised so many of you had no idea about the glass rod thing. It's quite unbelieveable because that's essential for doing laboratory
work. 
We learned these things as kids in elementary schools, along with making round/fluted filter papers, using a cork borer and cutting glass tubing. It's
in every elementary school chemistry textbook and repeats itself in highschool and college manuals.
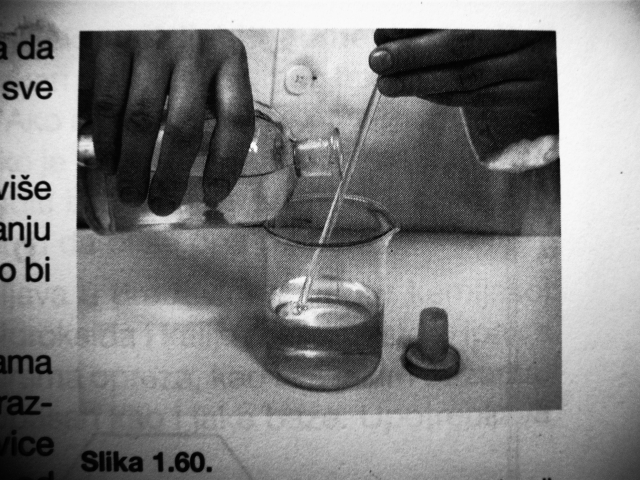
Every lab I've ever worked in has like a ton of these rods lying around.
Of course, it doesn't work for mercury and works poorly for bromine, obviously because of their atomic/molecular structure. Water is very polar, so it
sticks to the glass nicely.
Seriosuly, invest in a laboratory manual. Buy the actual book. Paper book, that's the one that is flammable. 
It's a lot better than downloading it.
You can't do chemistry while being educated over the Internet. I own few manuals and I assure you, things you can find over the Google are less than
10% of the stuff you can learn from manuals.
|
|
|
| Pages:
1
2 |