| Pages:
1
2 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Low density and pyrophoricity apart from the expense militates against its use,
but the ultimate hydrogen gas propulsion reaction would use straight liquified
Diborane in a hybrid arrangement having the Diborane tank over the Bifluoride
matrix combustion cavity.
2 B2H6 + 6 NH4F.HF -> 4 BF3 + 3 N2 + 21 H2
2 : 7, molar expansion 75 % Hydrogen by volume , 10.3 % by weight
Due to the very low melting temperature of Bifluoride 124º C ts hard to see how this
would remain solid for very long despite ablation from surface burning. It could
more easily be used premelted in lquid phase. As I pointed out before in my other
post above in this thread, " cryogenic fuel schemes have that much less latent
heat than a fuel at ambient temperature and this must be made up from the
energy provided by burning. " As a melt , heat energy is added to the system.
______________________________________
I had posted this on a similar scheme here
http://www.sciencemadness.org/talk/viewthread.php?tid=8678&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=8678&a...
_____________________________________
U P D A T E
The density of NaBH4 is barely more than water only 1.07 and it melts at just under body
temperature at 36º C. The shortcomings of using this as a solid are much more pronounced
than for NH4F.HF. It does however compact a large quantity of Hydrogen gas, its chief merit.
It can be readily melted and used in a liquid form. This has the advantages of increasing the
latent energy of the system, simplifying the layout, affording throttle-ability, safe storage
without risk of spills ( the fuel components are then solid ) and unlike many other energetic
chemicals these cannot explode by themselves.
2 NH4F.HF + NaBH4 -> NaF + BF3 + N2 + 7H2
3 : 10 , molar expansion 70 % Hydrogen by volume , 9.2% by weight
A combustible exhaust makes the the rocket plane concept a better approach than direct
verticle lift, since this applies the concept of the inclined plane to aeronautics. Supplemented
by combustion with air, the thrust can be varied so there is little initial consumption of fuel
from takeoff until a high altitude is reached, and then the full propulsion can be engaged to
acheive trans-atmospheric altitudes.
Discovered in 1943 by H.C. Brown, one of boron chemistry's Nobel prizewinners, and
H.I. Schlesinger, Sodium Tetrahydroborate ( NaBH4 ) proved to have strong possibilities
as a missile propellant. By far the world's leading commercial producer of NaBH4 is
Morton International Inc. Long famous for table salt, Morton is also a major manufacturer
of specialty chemicals. The process involves reacting Boric acid with Methanol to produce
Tri-Methyl Borate ( B(CH3O)3 ) which is then reacted with Sodium Hydride at elevated
temperatures. This yields Sodium Borohydride and Sodium Hydroxide together. It is soluble
in Methanol and also Water at 550 gm / Liter , decomposing slowly unless the solution is
basic, which is why it is contained with lye.
See this video of Tri-Methyl Borate - http://exploscience.com/Home/Green Fire Light.wmv
Powerpoint presentation
http://gcep.stanford.edu/pdfs/hydrogen_workshop/Wu.pdf
Pricey
http://www.usbweb.com/category.asp?special=&cat=bio&...
http://store.hvchemical.com/search.htm?step=2&viewfrom=1...
http://www.gfschemicals.com/chemicals/gfschem-A7158.asp
[Edited on 6-11-2007 by franklyn]
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
| Quote: | cryogenic fuel schemes have that much less latent
heat than a fuel at ambient temperature and this must be made up from the energy provided by burning. |
It doesn't all have to come from the burning. That is what regenerative cooling, running the propellant through pipes or cavities in the nozzle and
chamber, is for. The propellant becomes a warmer supercritical fluid while the nozzle and chamber are cooled. Remember cryogenic LOX is one of the
most successful liquid oxidizers, while cryogenic liquid hydrogen is one of the highest impulse liquid fuels, despite its great bulk and low density.
Diborane too would be a chilled fuel, with its critical temperature of 16.6°C, though it would be more practical (in terms of vapor pressure) to
store it in a pressurized dewar at like -35°C. I think it would work just fine despite its low density, much like liquid hydrogen works just fine.
Diborane is relatively easy to synthesize too (from NaBH4 or NaH), but it's definitely hard to handle. It can be made from BF3 and NaH or NaBH4, or
by acidifying or halogenating NaBH4.
The video you linked doesn't work. Bandwidth Limit Exceeded. I thought the
borohydride synthesis yielded borohydride and methoxide (or ethoxide, if you used triethylborate) though, not hydroxide. Bandwidth Limit Exceeded. I thought the
borohydride synthesis yielded borohydride and methoxide (or ethoxide, if you used triethylborate) though, not hydroxide.
B(OCH3)3 + 4NaH --> NaBH4 + 3NaOCH3
Only after addition to water would this react to form sodium hydroxide, but sodium borohydride reacts with water. Wikipedia describes
recrystallization in diglyme as an appropriate method for isolating the borohydride.
[Edited on 1-11-2007 by kilowatt]
The mind cannot decide the truth; it can only find the truth.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
One hand washes the other so to speak, quenching the laval nozzle allows a
lighter engine that won't melt, a cryogenic fluid however introduces material
problems due to thermal shock. Few alloys can withstand that differential. A
hot fluid imposes less of a constraint on the metal properties.
I know side reactions in the formation of NaBH4 are more complex , I don't have
a description of the proces.
An alternative :
3 NaBH4 + AlCl3 -> 3 NaCl + Al(BH4)3 , Aluminum Borohydride is not stable and
breaks down into an uncoordinated eutectic Al(BH4)3 -> AlH3 + 3BH3
Renewed interest in AlH3
http://stinet.dtic.mil/cgi-bin/GetTRDoc?AD=A441121&Locat...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Whole lotta shakin goin on
I archived this a few years back, I had considered using Hydrogen rich exhaust
from a hypergol propellant for fuel to power a compound rocket turbine engine.
The idea was that a turbo-supercharger could be simply modified so that the
propellant will spin the turbine and the exhaust can be further combusted with
the air from the compressor. The fuel would need to be liquid to be throttled
and cheap and available limiting any exotic component to a minimum. I settled
on 8 parts Ammonium Nitrate in solution in one part water at the boiling point
as the oxidizer and because it is also Hydrogen rich. The reducing component
would also need to be liquid and react on contact with the Ammonium Nitrate.
The criteria of Hydrogen rich and pyrophoric is satisfied by Diborane , limiting it
to a minimum 12.9 % weight of the total by solvating it with Ethylene Diamine
also very Hydrogen rich , H2N-CH2-CH2-NH2 : B2H6 . Only the Boron and Carbon
consume available oxygen the exhaust is 2/3 Hydrogen by volume 9.9% by weight.
Carbon Monoxide increases the combustable volume by 12.5% for a total 78.7 %
. . . . . . . . . . . . . . . . . . . . . Ratio of moles of reactants -> .
1 0 2 .:. 5 1 2 . -> to moles of products . . . . . . . . . . . . . // . . . combusted in air
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . / . . . . . \
3 2 .H2N-CH2-CH2-NH2 : B2H6 .+. 4 5 NH4NO3 .+. 2 5 H2O . => . 3 2 B2O3 .+. 6 4 CO .+.7 7
N2 .+. 3 3 9 H2 . // . 2 0 2 O2 .+ . 8 0 8 N2
. . . . . . . . . . . . . . . . | . . . . . . . . .| . . . . . . . . . . . .| . . . . . . . . . . . | . . . . . . . . . . .
. | . . . . . . . . . . | . . . . . . . . | .
. . . . . . . . | . . . . . . . . . . . | . . . . . . . . . . |
molar mass . . 1920 . . . . . 883 . . . . . . . .
3600 . . . . . . . 450 . . . . . . . . . .2227 . . . . . .1792 . . . .2156 . . . . . . 678. . . . . . . . 6464 . . . . . 22624
| Quote: |
I have subsequently revised this to exclude Diborane on the grounds that
it is self igniting and a safety hazard as well as not meeting the cheap and
readily available criteria. A gel of dry Isopropanol and Aluminum containing
Isopropoxide formed first in situ as the ignition source is by far more practical.
http://en.wikipedia.org/wiki/Aluminium_isopropoxide
The alcohol itself provides some oxygen so less Ammonium Nitrate is required.
I'm toying with the idea of a combined Ammonium Nitrate , Ammonium Bifluoride
melt. Apart from Ammonia contained , this same formulation was used for IRFNA
( Inhibited Red Fuming Nitric Acid ) passivated with 0.6 % Hydrogen Fluoride
http://www.astronautix.com/props/nitdudmh.htm
|
The diagrams below show various schematic and cutaway views of ordinary
turbosuperchargers. The only components are the the exhaust turbine and
compressor impellor mounted at opposite ends of the common shaft , the
central journal houses the full floating bearing , and also mounts the two
rotor housings called volute scrolls. My design excludes the compressor's
housing and envelopes the entire turbo unit instead with a cowling somewhat
like a nacell of a jet engine containing the aspirated air which is directed over
the turbine's housing to mix with the exhaust at that end. Additional air and
thrust bearing is provided by the trailing augmentor section. - Center diagram
An intriguing innovation is this air-bearing which eliminates the need for oiling.
http://www.miti.cc/newsletters/06_oilfree_turbocharger_gas_e...
.
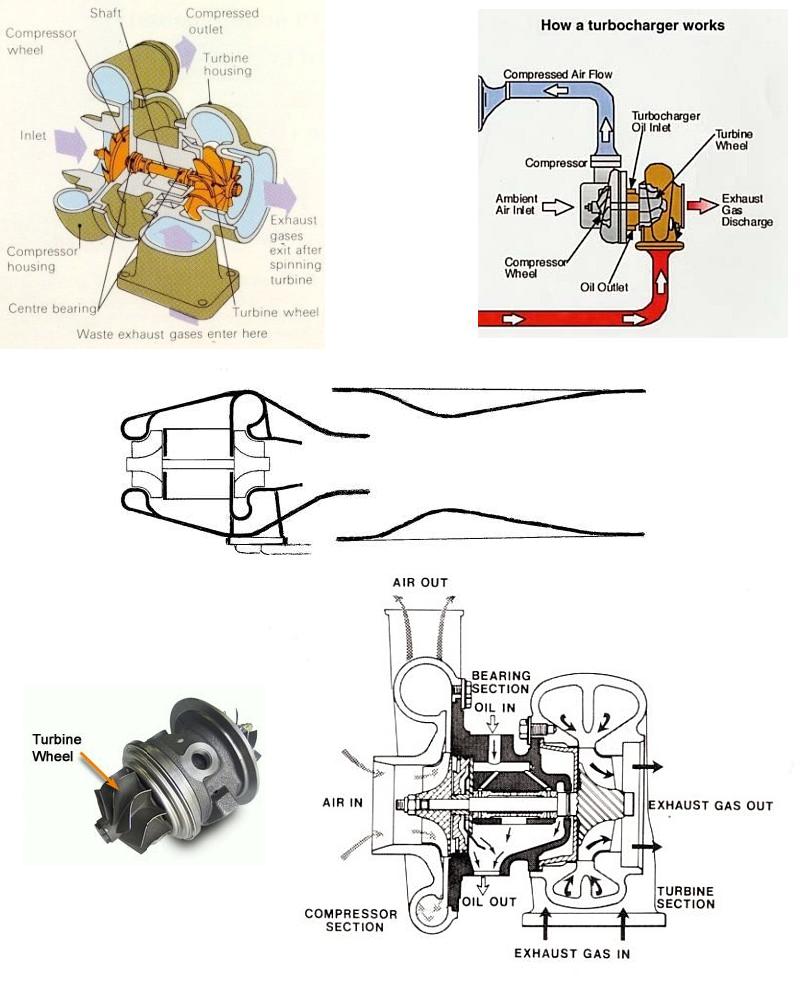
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
...
2 B2H6 + 6 NH4F.HF -> 4 BF3 + 3 N2 + 21 H2
8 : 28 , molar expansion 75 % Hydrogen by volume , 21.3 % by weight
... |
This reaction is slightly endothermic, H~+202kJ 
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
- artem -
has posted above observing that the " propellant " mix I proposed is in fact
endothermic. We exchanged U2U's over this and he remains steadfast in
his conviction, as I do that his assertion flies in the face of common sense.
( I have subsequently corrected the 21.3 % to 10.3 , which was in error )
The maddening thing about this is that when one does the requisite
assigning of enthalpies ( cited in references ) to the balanced equation
there appears to be a large discrepancy over what the result is.
[ artem ] has given me two different figures , and every time I do the
math ( using cited references ) I get a different result - Grrr.
The equation of the reaction is _ 2 B2H6 + 6 NH4F.HF => 4 BF3 + 3 N2 + 21 H2
B2H6 has a Positive heat of formation , is pyrophoric and spontaneosly
burns in air , inherently acidic NH4F.HF de-oxidizes Aluminum !
It is elementary that acids energetically replace hydrogen from hydrides.
The notion that combined , these will form a cold pack is facetious.
Granted that HF has a high enthalpy to begin with , and so less energy is
available. This is similar to a reactive metal with a chlorocarbon , energy
will be less than if the metal is oxidized with chlorine directly because the
carbon is also being reduced.
2 B(s) + 3 F2(g) -> 2 BF3(g)
. . . . . . . . . . . . . . . . . . . -2272 ( -1136 KJ / mol )
B2H6(liq) + 6 HF(g) -> 2 BF3(g) + 6 H2(g)
+ 41 . . . . . . . . .-1638 . . . -2272 . . . . . .
. . . - 675 KJ reaction enthalpy
I have assumed dis-association of 2 NH3 into N2 + 3H2 . Enthalpy of NH3 is
- 46 KJ / mol , so in reverse this is endothermic , but still the molar volume
increaes ( opposite of the Haber process for its formation by Le Chatelier ).
Assuming double the above value -1350 and subtracting the heat value of
the 6 moles of ammonia -276 KJ corresponding to the balanced equation with
Amminium Bifluoride , leaves -1074 KJ which appears a reasonable value.
In my conception NH4F.HF is to be in a liquid phase for use , as I pointed
out in a previous post , the lattice energy and heat of fusion , is thus
added to the enthalpy. BF3 is a gas so the heat associated with the phase
changes to liquid and then solid , remains in the substance itself and for this
reason is not evident in the accounting , this endothermic component is not
expressed in the figures.
The solution otherwise is uncomplicated and straight forward :
http://pages.prodigy.net/anderhan/ch11thermo.pdf
Quote _
" Enthalpy changes for reactions can be obtained by simply subtracting the
heats of formation of the reactants from the heats of formation of
the products. Be sure to multiply the heats of formation by the coefficient
of the compound involved."
Also , arithmetically subtracting a negative is the same as adding a positive.
The big problem is obtaining consistent enthalpy values. There is aparently no
agreement , convention , consensus , consistency or standard as to exactly
what constitutes " standard " values although it may be stated as such.
I don't quibble with the units , that is easily convertable , but the fact that
it may be calculated at absolute zero 0º K or 298º K ( 0º C ) or often
at an arbitrarilly chosen phase without stating so.
For example , given in 0º Kelvin > http://srdata.nist.gov/cccbdb/hf0k.asp
From > http://cobweb.ecn.purdue.edu/~propulsi/propulsion/comb/prope...
( Fiqures are given in calories / gram , this I first multiply by the molar mass
and and again by 4.184 to convert into Kilojoules / mol )
B2H6 is + 354 cal / gm = ( + 40.9 KJ / mol , liquid
NH4F.HF is -3189 cal / gm = ( -760.5 KJ / mol
NH4F is -3000 cal / gm = ( -464.4 KJ / mol
NH3 is -649 cal / gm = ( -46.2 KJ / mol
HF is -3581 cal / gm = ( -299.6 kj / mol , liquid
BF3 is not indicated
From > http://webbook.nist.gov/chemistry/form-ser.html
( substances must be individuallly researched )
B2H6 is + 41 KJ / mol , liquid
NH4F.HF is not indicated
NH4F is not indicated
NH3 is -45.9 KJ / mol
HF is -273.3 kJ / mol
BF3 is -1136 kJ / mol
From > http://www.ualberta.ca/%7Ejplambec/che/data/p00403.htm
B2H6 is not indicated
NH4F.HF is not indicated
NH4F is not indicated
NH3 is -46.1 KJ / mol , gas
HF is -271.1 kJ / mol , gas
BF3 is -1137 kJ / mol , gas
From > http://www.grc.nasa.gov/WWW/CEAWeb/TP-2001-210959-REV1.pdf
B2H6 is + 36.6 KJ / mol , gas
NH4F.HF is not indicated
NH4F is not indicated
NH3 is -45.9 KJ / mol , gas
HF is -273.3 kJ / mol , gas
BF3 is -1136 kJ / mol , gas
From > http://www.update.uu.se/~jolkkonen/pdf/CRC_TD.pdf
STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES
B2H6 is + 36.4 KJ / mol , gas
NH4F.HF is not indicated
NH4F is -464 KJ / mol , crystal
NH3 is -45.9 KJ / mol , gas
HF is -299.8 KJ / mol . liquid <> -273.3 kJ / mol , gas
BF3 is -1136 kJ / mol , gas
From - CRC Handbook of Chemistry & Physics
B2H6 is + 36.4 KJ / mol , gas
NH4F.HF is not indicated
NH4F is -464 KJ / mol , crystal
NH3 is -45.9 KJ / mol , gas
HF is -299.8 KJ / mol . liquid <> -273.3 kJ / mol , gas
BF3 is -1136 kJ / mol , gas
From - Kirk Othmer encylopedia
( substances must be individuallly researched )
B2H6 is + 35.5 KJ / mol , gas
NH4F.HF is -798.3 KJ / mol , crystal
NH4F is -466.9 KJ / mol , crystal
NH3 is -46.2 KJ / mol , gas
HF is -272.5 kJ / mol , gas
BF3 is -1135.6 kJ / mol , gas
Heat of formation values used for NH4F.HF
( The value -464 for NH4F(s) , and -273 for HF(g) are combined , -737 )
B2H6(liq) is + 41 , NH3(g) is -46 and BF3(g) is -1136
2 B2H6(g) + 6 NH4F.HF(l) -> 4 BF3(g) + 3 N2(g) + 21 H2(g)
. .+ 82 . . . . . . . .-4422 . . . . . . .
-4544 . . . . . . . . . . . . . . . . . . . - 204 KJ reaction enthalpy
Alternatively _
2 B2H6(g) + 6 NH4F.HF(l) -> 4 BF3(g) + 6 NH3(g) + 12 H2(g)
. .+ 82 . . . . . . . .-4422 . . . . . .
.-4544 . . . . . -276 . . . . . . . . . . . .- 480 KJ reaction enthalpy
One way around this provides only an approximate result , and it is not
endothermic. Summing the individual average bond energies of the reactants
and the products > http://www.cem.msu.edu/~reusch/OrgPage/bndenrgy.htm
REACTION _ 2 B2H6 + 6 NH4F.HF -> 4 BF3 + 3 N2 + 21 H2
BOND . . . . .H - B . . . H-N . . . H-F . -> . B-F . . . .N2 . . . .H2
ENERGY . . . 90 . . . . . 93 . . . 135 . .-> .150 . . . 226 . . . 104
COUNT . . . X 12 . . . X 18 . . .X 12 . -> . X 12 . . . X 3 . . .X 21
TOTALS . . .1080 . . 1674 . . 1620 . -> .1800 . . 678
. . 2184
- 288 KJ reaction enthalpy
Well there you have it every way I calculate it from worst case it comes up
exothermic. What latent heat might be present in NH4F.HF , is the variable.
________________________________________________________
Diborane was considered in the 1950's as a fuel but never deployed.
This may not be a practical propellant due to the errosive nature of HF,
teflon lined non-metal fittings would be needed at least. It is in keeping
with this thread in exploring beyond the current art.
http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=30
NOTE you must copy this URL and paste into the address bar
Most energetic reaction ?
http://www.math.temple.edu/~wds/homepage/chem.records
Here is research with OF2 as an oxidizer for Diborane
http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/1970000...
Turbojet combustion of Diborane
http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/1993008...
.
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
...Heat of formation values used for NH4F.HF
( The value -464 for NH4F(s) , and -273 for HF(g) are combined , -737 )
...Well there you have it every way I calculate it from worst case it comes up exothermic. What latent heat might be present in NH4F.HF , is the
variable.
|
Well, franklyn
1)NH4HF2(s) H0=-804KJ/mole(Chem.Encyclopedia)
(it is solid - Tm=+126.45C)
your mistake is "combining" without the enthalpy of
sublimation HF -36.4KJ at 292.7K) and
reaction HF(l)+NH4F(s)=>NH4HF2(s), H~-28KJ/mole
Another figures for NH4HF2 (-798 KJ, for example) are possible, but the the difference is negligible.
(figure -760.5 for NH4HF2 may be wrong, such internet sources often contain mistakes)
2)the reaction B2H6+NH4HF2 may be exothermic:
0.5B2H6(g)+3NH4HF2(s)=>NH4BF4(s)+2NH4F(s)+3H2+~295KJ
2.5B2H6(g)+3NH4HF2(s)=>3BN(s)+2BF3(g)+15H2+~704KJ
and so on...
but it is BAD PROPELLANT anyway, it is suitable only as H2-source.
3)NF4HF2 is more suitable for propellant 
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
LA-2341 - The performance of boron explosives
Available from the First CD of the Los Alamos monograph collection
This 1959 study of mixtures of Boranes with various Fluorocarbons showed that
even in reducing carbon performance is only slightly inferior to TNT. Notably Boranes
were also mixed and exploded with only Hydrazine and that is not even considered to
be an oxidizer yet obtained again performance slightly less than that of TNT yielding
Boron Nitride and Hydrogen.
.
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
This 1959 study of mixtures of Boranes with various Fluorocarbons showed that even in reducing carbon performance is only slightly inferior to TNT...
. |
Theory gives 5.9MJ/kg for teflon+B5H9,B10H14 (and ~110% blast effect vs TNT)
See also US Patent 5487798 - using mixtures of NH4N3+B,Ti,TiH2.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by hinz  | Learn some basics of rocketry first, if your Mg/KMnO4 propellant won't explode, you would melt your rocket case down at the temperatures Mg burns, and
the MgO produced will stay inside your molten case or sinter on the parts of the nozzle, where the pressure is decreased (the bell shaped end). The
nozzle would be tortured by liquid Mn and the trust wouldn't be good as most of the producs of the reaction (MgO) won't leave the nozzle, only the K2O
and the liquid Mn would leave it and generate trust.
There is a reason why Werner von Braun has invented the liquid propellant rockets, because those designs you make won't work as no material will hold
up the temperatures involved without beeing cooled.
With liquid propellants you can cool the nozzle with propellant and cheaper and easy handable propellants like kerosene/LOX can be choosen without
decrease in reaction enthalphy and thus trust.
If you want to mess around with metall hydrides (your last crazy idea), fist look at their properties you you don't blow yourself up in a clowd of
hydrogen (because your propellant got sligtly wet) as you ignite the fuze. (supposed you even get some metall hydrides)
If you still wan't to play with rockets, start with low tech KNO3/sugar,
here are some good pages for you:
http://www.jamesyawn.com/index.htm
http://www.nakka-rocketry.net/index.html (look at the rocket theory) |
Liquid Mn and K2O does not generage thrust.
Quote: Originally posted by APCP  | | Quote: | Originally posted by kilowatt
I also have a larger solid fueled aerospike in the works, which will use Al/NH4NO3 composite propellant.
[Edited on 28-10-2007 by kilowatt] |
ANCP chuffs when using Al. Unless you can get some cenes, you'll want to use Mg instead of Al.
Be would be your best bet for high performance metal.
You start reaching the upper limits of solids Isp when you use HNF/GAP. Expensive as hell, hard to get, hard to make....
Still, good luck on any experimentation. Get some diagrams for your aerospike designs made up, I am intrigued. My team plans on using an aerospike on
an R motor for a space shot. Probably won't because they are pain, but one varient of the motor has an aerospike. |
Use Magnesium for nitrates, aluminum for perchlorates.
Magnesium boils easily and react fast, aluminum boils 2600 which a nitrated propellant unlikely to reach, so al will react at its molten stage which
is not stable so it chuffs.
___________
and all those other comments are very nice, Lithium boro hydride might be the might hydride to use but its really unsafe,
the most practical might come to Magnesium hydride.
Or a composite propellant with dissolve oxidizier in a binder that releases flourine mixed with magnesium powder or hydride, magnesium burns really
hot with flourine, and for hottest chemistry reaction ever is lithium + flourine which combust at around 5000 degree.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
What material would be able to withstand that kind of thermal shock and still not fail? Diamond?!
|
|
|
| Pages:
1
2 |
|