| Pages:
1
2
3 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Today, I tried oxidizing 4-MeOBnOH with solid Ca(OCL)2 and TBAB in DCM.. The reaction proceeded very slowly (at 4H TLC indicated a lot of unrecated
alcohol, aswell as two impurities plus the aldehyde), and was IMPOSSIBLE to filter before workup. I tried everything I could, gravity filteration
before vcauum, large pore gravity followed by small pore, layer of Na2SO4 (I don't have celite around) on vacuum... Nothing would get rid of the tiny
particlues in suspension, that would immediatly plug any filter paper.
After 5H trying to get it cleared, I abondoned, and left it over a buchner with a certain amount of sludge. Very very slowly, it was passing through,
but I guess most of the DCM will evaporate before a decent amount of solution passes through.
So TCCA/KBr will be trie don the same substarte tomorow. Unfortunaly, I've only got 2 more days of relative free time, I will start deplacing myself
for work on thursday, and won't be able to spend much time in the lab. I've been working on these oxidations for neraly two weeks now, and have yet to
found a efficient, high yielding procedure; benzylalcohol oxidation to the aldehyde is most of the time considered trivial, and easy, I can't disagree
more. And authors of publications lie, or live in wonderland, a place where organic chemstry is a easy a typing a few pages on the computer. That's
the only explanation I can find.
As the result of my relentness, I've only worked on those damn oxidations, though I had lots of other reactions I had planned doing... Too bad...
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Your feelings are well justified, many of the published methods aren't any good. It may very well be that the authors of such papers have indeed seen
the products when running a sample of the reaction mixture through a GC-MS or whatever, but they might've omitted some nasty details or added 30% to
the yields. It might be that the reaction only works on microscale or that preparative use requires the use of chromatography for purification. It is
left to the reader to notice how the paper avoids less reactive or otherwise complicated substrates and how they do not discuss the problems they've
had to deal with at all.
This is why reading journals like Organic Process Research and Development is so great. How to solve the problems relating to a multikilogram
synthesis route is often the whole point of a long article for those who research such things! For them reactions that never fail are golden and
mechanical operations like extractions, washes, filtrations and such, that are often so easy and trivial for common laboratory chemists, are expensive
and to be made as fast and simple as possible, if not eliminated completely. On the other hand there are synthesis methods that give hundreds of hits
when you do a reaction search on a database. Most often there is a reason for why they are so popular.
Patents are often considered to be filled with inaccuracies or even lies, but at least the usual reason is that something indeed works, whereas the
lies of short Tet. Lett. papers are because of the opposite. You just have to learn to be suspicious and critical.
In my opinion the use of any halogen based oxidation system with aromatic substrates that are activated towards electrophilic substitution, such as
p-methoxybenzyl alcohol, is a bad idea since you are likely to get a lot of ring halogenation.
If I were you, I'd have a look at US4146582, the following thread and the articles I have put online:
https://sciencemadness.org/talk/viewthread.php?tid=410&p...
Notice how these free radical chemists have published their invention both in a journal and a patent, and how their economic incentive was the ability
to oxidize p-methoxytoluene to the benzaldehyde selectively in presence of the meta-isomer. From their point of view using this reaction for oxidation
of p-methoxybenzyl alcohol is trivial. However, I would be very surprised if phenols (p-cresol for example) worked as substrates.
Good luck!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you trilobite, for the article, I wonder why I didn't stumble on them before. A few years ago, I did alot of experimenting with theis
Cu2+/Fe2+/S2O82- system, both with toluene and benzyl alcohol. Not once did i manadged to get a decent yield of the aldehdye, although it smelled
strongly of almonds. The brown/red organic phase after the 2hours of reaction had alot of impurities, benzoic acid was present at all times, and was
some times the major product (very large cristals on cooling). I had put alot of time and effort in the method (and alot of reagents), and just
finished by forgetting about it.
The only information I had back then was the patent, which obviously sounded very good. I used Cu(OAc)2 and FeSO4.7H2O as salts, and 99.9% lab grade
ammonium peroxodisulfate (more soluble than the K persulfate).
Have you tried out this method? (not the Mn persulfate) What where your results if any? I'd love to hear some succes stories about it, the reaction
itself was quite simple and easy (never got any runaway, although I used 600mL max total solution).
I think I'm going to try freshly precipitated MnO2, at a small scale, although from what I heard it's a bitch to work with...
BTW, benzaldehyde itself isn't really a major concern to me, I've got a bottle of lab grade and not much uses to it, it's more about finding generally
oxidation method, that will equally work with substitued benzaldehydes, but I'm not going to waste expensive/rare substrates before hand! 
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Very interesting! One of my questions back when I tried to find whatever research that had been done with free radical oxidations like these was
whether you could use (NH<sub>4</sub> <sub>2</sub>S<sub>2</sub>O<sub>8</sub>. I myself have never tried the reaction, but have been planning to try it
out when I get the chance. The reason for my enthusiasm is that the mechanism is quite well established by several authors, but the patent is where
these principles are applied to practice. You may have the references if you wish so. It is easy to understand why you may not be as enthusiastic,
though. Here is what I think could have been the problem. <sub>2</sub>S<sub>2</sub>O<sub>8</sub>. I myself have never tried the reaction, but have been planning to try it
out when I get the chance. The reason for my enthusiasm is that the mechanism is quite well established by several authors, but the patent is where
these principles are applied to practice. You may have the references if you wish so. It is easy to understand why you may not be as enthusiastic,
though. Here is what I think could have been the problem.
First toluene is oxidized to a radical cation by a sulfate radical, the radical cation loses a proton and becomes a benzyl radical. And now the
important part: Cu<sup>2+</sup> ion oxidises the benzyl radical further in a one electron oxidation step, and one of ligands coordinated
to the metal end up on the benzylic carbon. Usually you'd expect to have a benzyl carbocation from such an oxidation, which would then react with eg.
water or some other nucleophile swimming nearby. But in this case one of the ligands coordinated to the copper ion acts as the nucleophile.
So if you have a lot of chloride ions in your solution, you get some benzyl chloride, high concentrations of acetate give some benzyl acetate and so
on. In the presence of ammonium ions opper ammine complexes can form, especially since relatively small amounts of copper are used. These complexes
could then cause formation of benzylamine as product. Benzylamine might be further oxidized to benzonitrile, or it might form an imine with
benzaldehyde which might react further some way. I guess this sort of reaction pathways could be possible also for benzyl alcohol. Of course they
depend on the pH of the reaction mixture, which decreases as the reaction proceeds.
Lower pH should also decrease the concentration of copper ammines. Actually ammonium peroxydisulfate has been used in some of the papers by other
researchers where this sort of reactions have been studied, but typically in an acetic acid or acetonitrile solution, not aqueous methanol. Usually
these articles focus on mechanistic studies, such problems might not show up when there is something like 0.2 or 1 equivalents of copper used in
relation to the substrate or oxidant. In the patent several solvents are mentioned, including acetic acid and acetonitrile, but no example is given
where these solvents or ammonium peroxydisulfate were used, so I guess they are there to claim as much as possible. The downside of using potassium
peroxydisulfate would be that it should either be added as a slurry (stirrer in the addition funnel? ) or portionwise as powder. This would be a rational explanation for why these guys seem to use the sodium salt all the
time So, maybe this could be behind your troubles. ) or portionwise as powder. This would be a rational explanation for why these guys seem to use the sodium salt all the
time So, maybe this could be behind your troubles.
Here are abstracts on similar reactions which give nitriles. They are all done in basic conditions, as they obviously should be.
<b>New synthesis of nitriles.</b>
Brackman, W.; Smit, P. J.
Recueil des Travaux Chimiques des Pays-Bas, 82(8), 757-62 (1963).
Journal written in English. CAN 59:75021 ISSN 0165-0513
<u>Abstract</u>
Nitriles were prepd. by stirring a methanolic soln. of an aldehyde, NH3, a complexed Cu salt, and a strong base under O. Under these conditions, MeOH
was slowly oxidized to give low yields of cyanide and cyanate. With MeOH as the only substrate, substitution of NH3 by a primary amine led to the
formation of small amts. of isocyanides. Thus, 100 ml. soln. of 4 millimoles CuCl2.2H2O, 400 millimoles NH3, 30 millimoles NaOMe, and 50 millimoles
BzH in MeOH at 30 Deg stirred 6 hrs. with O gave 66 PhCN. The yield was 79% when the reaction time was 23.5 hrs. Other nitriles prepd. were the
following (compd. and % yield detd. by mass spectroscopy given): p-MeOC6H4CN, 82; 3,4-methylenedioxybenzonitrile, 82; 4,3-HO(MeO)C6H3CN, >40;
o-O2NC6H4CN, 51; 2,6-ClC6H3CN, >76.5; 2-cyanofuran, <10; CH2:CHCN, trace; EtCN, 18; PrCN, 28; Me(CH2)5CN, 63, cinnamonitrile, 55. Aromatic
alcs. were also employed, but the yields were low. H2O retarded the reaction. A mechanism involving the oxidn. of aldimine to an imine radical by the
Cu(II) complex as the rate-detg. step was proposed.
See also patents DE1169937 and GB920987
<b>A catalytic synthesis of nitriles from aldehydes and alcohols in the presence of aqueous ammonia by oxidation with NiSO4-K2S2O8.</b>
Yamazaki, Shigekazu; Yamazaki, Yasuyuki.
Chemistry Letters, (4), 571-4 (1990).
Journal written in English. CAN 113:58637 ISSN 0366-7022
<u>Abstract</u>
Arom. and conjugated aldehydes and alcs. were converted to nitriles by nickel-catalyzed oxidn. in the presence of aq. NH3 with K2S2O8 under the basic
aq. conditions. Thus, PhCHO was treated with K2S2O8 in the presence of NiSO4 in H2O-NH3 to give 76% PhCN. Similar treatment of PhCH2OH gave 82%
PhCN.
<b>Tetrabutylammonium peroxydisulfate in organic synthesis. Part 8. An efficient and convenient nickel-catalyzed oxidation of primary amines to
nitriles with tetrabutylammonium peroxydisulfate.</b>
Chen, Fen-Er; Peng, Zuo-Zhong; Fu, Han; Liu, Ji-Dong; Shao, Lan-Ying.
Journal of Chemical Research, Synopses, (12), 726-727 (1999).
Journal written in English. CAN 132:92845 ISSN 0308-2342
<u>Abstract</u>
Primary amines were oxidized to the corresponding nitriles in excellent yields with tetrabutylammonium peroxydisulfate catalyzed by nickel copper
formate under basic aq. conditions. Thus, treatment of MeCH2CH2CH2NH2 in ClCH2CH2Cl with (Bu4N)S2O8, aq. Cu(HCO2)2/Ni(HCO2)2, and aq. KOH at room
temp. for 10 h gave 92% MeCH2CH2C.tplbond.N.
<b>Tetrabutylammonium peroxydisulfate in organic synthesis. Part X. An efficient nickel-catalyzed one-pot synthesis of nitriles from aldehydes
by oxidation with tetrabutylammonium peroxydisulfate.</b>
Chen, Fen-Er; Fu, Han; Meng, Ge; Cheng, Yu; Lu, Yin-Xiang.
Synthesis, (11), 1519-1520 (2000).
Journal written in English. CAN 134:29005 ISSN 0039-7881
<u>Abstract</u>
Various aliph., arom. and heterocyclic aldehydes were efficiently transformed to the corresponding nitriles in a 1-pot procedure by Ni-catalyzed
oxidn. with (Bu4N)2S2O8 in the presence of NH4HCO3 under basic aq. conditions. The process affords excellent yields of pure nitriles.
<b>Tetrabutylammonium peroxydisulfate in organic synthesis. XIII. A simple and high
ly efficient one-pot synthesis of nitriles by nickel-catalyzed oxidation of primary alcohols with tetrabutylammonium peroxydisulfate.</b>
Chen, Fen-Er; Li, Yong-Ye; Xu, Mei; Jia, Hui-Qing.
Synthesis, (13), 1804-1806 (2002).
Journal written in English. CAN 138:204807 ISSN 0039-7881
<u>Abstract</u>
A facile 1-pot method is presented for the synthesis of nitriles from the corresponding primary alcs. by Ni-catalyzed oxidn. with tetrabutylammonium
peroxydisulfate in the presence of ammonium hydrogen carbonate under basic aq. conditions. This convenient synthetic method provides an easy and
simple access to various aliph., arom. and heterocyclic nitriles in excellent yields with very high purity.
I also stumbled upon this article that discusses the oxidation of alcohols with MnO<sub>2</sub> prepared by thermal decomposition of
manganese carbonate or oxalate. Usually it is assumed that the oxide needs to be prepared by reduction of permanganate The reference is Journal of
Organic Chemistry, 19, 1608-1616 (1954).
Keep up the good work!
Attachment: JOC_1954_1608-1616.pdf (566kB)
This file has been downloaded 1288 times
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Very interesting interpretation, must I say! I didn't consider any side reaction with the ammonium counter ion back then, as I only had my own
interpreation of the mechanism.. At the end of the oxidation, pH was very low though. Copper acetate was used in the proportions mentionne din the
patent, and they claim ammonium persulfate can be used, it's even in the prefered oxidants IIRC. But even with ammonium persulfate, huge amounts of
aq. soltuion are needed for a relatively small amount of substarte. Seeing that the difference of solubility at 20°C and 70°c is huge, I compensated
by adding half the oxidant as a saturated solution in H2O (MeOH only decreases the solubility, I have no idea why they add some in the patent), and
the other half as a solid. This was quite messy, especially the saturated solution that would clog the addition funnel as it cooled down (dissolution
is very endothermic).
With all this litterature behind, I have to say it revives my interest in this reaction. At a small scale it could be rewarding. It doesn't scale up
very well though, I remember story of arm-thick fountains spraying from side necks! IIRC, heating up to 70°c before adding most of the oxidant was
necessary...
In any case, I won't be able to work on it right now, today is my last day of lab work before a few weeks at least... But if you have the
possibility, please try it out by ourself, I'd be willing to share all my notes on theses reaction (when I found them).
And now, a beautifull little story:
Depressed as I was of having spent 10 days on a oxidation without get yields higher than 30%, I was going to abandon TCCA oxidations for good
yesterday. Finally, out of shear hope, I decided on doing one last oxidation, by the paper, of another substitued alcohol...
TCCA/KBr oxidation of 3,4-dimethoxybenzyl alcohol
In a 250mL 4-neck RBF, with a stir bar and thermometer, 8.41g (50mmol) of 3,4-MeOBnOH and 0.71g (6mmol) of KBr were introduced, followed by 100mL
DCM. A condenser was attached, and slow stirring started.
Roughly 3g of fresh silica gel (1) were grinded in a fine powder, and then flooded with dH2O until no more bubbles evolved. The suspension
was thne vacuum filtered, and the white powder scrapped of after 30 sec of suction on the buchner. 5.4g of the wet silica were introduced in the
reaction flask, which gave a snow-flake-like suspension.
5.89g (23.28mmol) of powdered TCCA were then quickly added. The color immeditaly turned yellow, and an exothermic reaction setted in (2),
the temp increasing to 35°c. A ice bath was immeditaly applied, and the temperature slowly fell back to 10°C. A crust of wet silica slowly formed on
the sides of the flask, and was regularily scrapped back into solution. The color became more and more prononced, towards orange. AT 30min, TLC
indicated only two spots, the alcohol and the aldehyde.
The reaction was monitered by TLC during 2 hours, another 3.34g (13.39mmol) of TCCA being added in portions. Only a small stain of alcohol remained,
aswell as a large aldehyde stain. The suspension was of a very dark red color now.
It was easily filtered, the remaining solids being extracted with 3x20mL DCM, until the extracts were only slightly yellow. The dark red soltuion was
dried over Na2SO4 for 10min, then decanted into a 250mL RBF, and the DCM removed under Argon, over a 55°c water bath. At the end of the distn, a
white solid started appearing in the condenser, insoluble in DCM, acetone or water. The last traces of solvant were removed with a slight vacuum, then
150mL of dH2O added to the dark brown residu, wich immeditaly solidified. Heating with a mantle was started, and the solids melted when ebulliton
started. Steam distn started, under Argon, but the organic distillate solidified in the condenser. The cooling water was stopped, and the distillate
ledt to heat up the condenser, until the solids were deplaced to the end of the condenser. The cooling water was replugged, and the remaining solids
melted with a flame to fall in the receiver. This was quite tricky, but finally I manadged to get it right. This was repeated for over 3H, until 250mL
distillate/ beautifull nice smelling white crystals were obtained. TLC confirmed that theses solids were pur (single spot) aldehyde! After a while,
the organic distillate seemed to diminish, and take a very slight yellow tint in the condenser. Steam distn. was stopped, the obtained solids crushed
up a little, and vac. filtered, washed with a little dH2O, dried under suction, and then in a CaCl2 dessicator over night. In the morning, they
weighed 6.20g (37.30mmol), 74.60% yield! And the distillate is yet to be extracted, aswell as the condenser! The reaction yeild must
ahve been over 90%, counting the losses from the delicate steam distn. (I could have continued it for another few hours, but was exausted)
succes!!!
So I publicly express all my apologies to Mohammed Ali Zolfigol and his team for having dismmissed their work and called then liars! THis is a great
piece of work, and my failures clearly demonstrate that minor changes can make huge difference, between total failure and success! I guess
chromatograpy-garde silica was employed in their trials, and the first batch of silica I had made didn't have sufficient pore size/number. If this
protocol worked with a delicate substarte, it will clearly work for other substitued benzyl alcohols.
Got it right at the last day! At least I know what I'll be doing during my next period of free time!
(1) This silica wasn't prepared as the first batch. It was left to polymerise nicely for 3H after total acidification of the sodium silicate
solution, and was much more thinner than the first batch. Apparently, this changed everything. The water content must have been higher, and much more
"accesible". The procedure from Lab Techniques in Organic Chemistry, B. Keil, 1966 (russian translation) was followed.
(2) This never happened before, in the other trials. That was the moment where I said to myself, "this might actually work out!". That means
that the TCCA generated much more HOCl than the other times, and most of it didn't react with the formed HCl to give Cl2. No Cl2 smell was present
during workup. In the other trials, the TCCA mostly stayed as is, and react immediatly with any formed HCl.
PS: Trilobite, thank you for the MnO2 article, but I wont be needing it right now!
EDIT: Steam distillation was started again, and, although the orgnaic distillate is considerably weaker, some solids are still passing. These are a
little less pur, have a slight yellow tint, and have tiny stains at the alcohol and another hardly eluted impurity. Nothing a good'ol
recrystallization can't take care of!
For the moment, at least another gram has been isolated, which roughly gives a isolated yield of 87%! And it's not finished! The total yield, after
recrysatllization of the last portion of aldehdye will be given as soon as available. This method is definatively worth the effort! Can't wait to try
it on other substitued benzyl alcohol!
I will try it again on p-MeOBnOH to see if any chloriantion accures, and maybe on p-NO2BnOH to see how bad the influence of the nitro is.
Conclusion:
Out of a series of oxidations using hypochlorite ( generated insitu or not), the combination of TCCA (trichloroisocynauric acid), KBr and wet SiO2
in DCM (M. A. Zolfigol et al. Syn., 12 (2006) 2043–2046) gave the most satisfying results. The quality of the silica gel and it's
preparation seems critical to obtain the high yields mentionned by the authors. The reaction worked cleanly and quickly on 3,4-dimethoxybenzylalcohol,
and is thus supposed to work on different not-too-desactivated substitued benzyl alcohols. Furhter trials on different substrates will be tried. This
method is thus a cheap, clean, and efficent way of converting benzylic alcohols to their corresponding aldehydes, with no, or little, over-oxidation
to the carboxylic acid. Amen.
[Edited on 7-11-2007 by Klute]
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Congratulations! I think I'll have a look at the reference. The effect of using silica that way is quite interesting. Have you verified that your
product is indeed veratraldehyde, except with TLC? The reason for my question is that distinguishing between halogenated benzaldehydes and their
parent compounds can be tricky with TLC. Chloroform seems to separate such cases where other solvents won't.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
GC confirmed 99,4% 3,4-dimethoxybenzaldehyde, straight after recrystallization!  The minor impurity is the starting substarte, wich seems to pass in very small amounts during steam distillation. The last batch, the less pur
crysatls, contained a bit more alcohol, and a very small amount of colored impurity that doesn't elute with DCM. After recrystalization, it was
impossible to differentiate between the two samples..
The minor impurity is the starting substarte, wich seems to pass in very small amounts during steam distillation. The last batch, the less pur
crysatls, contained a bit more alcohol, and a very small amount of colored impurity that doesn't elute with DCM. After recrystalization, it was
impossible to differentiate between the two samples..
The total dried wait of the isolated solids thus sums up to 6.75g (40.62mmol) 81.24%!
I haven't distilled the DCM extracts of the aq. distillate yet, I hope I can get another 0.1-0.3g out of it...
Can't wait to try the reaction on another substarte, but unfortunaly, it's not until a little moment...
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Now that's what I wanted to hear!  If you ever want to test the limits of this
method, then 2-hydroxybenzyl alcohol, vanillyl alcohol, 3,4-dihydroxybenzyl alcohol and maybe 2,5-dihydroxybenzyl alcohol should be simple but tricky
substrates with activated rings, possibility for acid-catalyzed polymerization and also oxidation to the quinone (dihydroxylated species). Interesting
curiosities, but maybe not the most practical substrates that one would try first. If you ever want to test the limits of this
method, then 2-hydroxybenzyl alcohol, vanillyl alcohol, 3,4-dihydroxybenzyl alcohol and maybe 2,5-dihydroxybenzyl alcohol should be simple but tricky
substrates with activated rings, possibility for acid-catalyzed polymerization and also oxidation to the quinone (dihydroxylated species). Interesting
curiosities, but maybe not the most practical substrates that one would try first.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Silica gel or celite supported Jones reagent looks interesting. The Cr seems to stay bound to the support.
(I'm just starting to look at this, so no references yet.)
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Haven't much time to elaborate, but it seems the recation yield only the corresponding chloride with another substitued substarte. I will have to
confirm the veratraldehdye is really what I thought it was. The two give positif bisulfite test, but the chloride could be hydrolysed to the aldehdye
wich then formes an adduct... A sommelet hyrdolysis will be tried, and if it yields an different product, we will be settled... The expected aldehyde
had a very high mp, corresponding suspiciously to the range of the chloride.. More news later...
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
For reactions using TCCA/ KBr ...Im assuming the active species formed is BrO-. Why is BrO- better than ClO-? Hypochlorite is a stronger oxidizer.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I think that's exactly why BrO- is prefered for benzylic alcohols, to avoid further oxidation. It must be smoother and more selective than
hypochlorite...
Haven't had time to test the product yet... Will let you know..
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Related to the TCCA methods:
| Quote: | Highly Efficient Catalytic Aerobic Oxidations of Benzylic Alcohols in Water
1 mol-% TEMPO and a catalytic amount of 1,3-dibromo-5,5-dimethylhydantoin and NaNO2 is a highly efficient catalytic system for the aerobic oxidations
of benzylic alcohols in water. |
http://www.organic-chemistry.org/abstracts/literature/845.sh...
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thanks for the link not_important, speaking of TEMPO, my 2,2,6,6-tetramethylpiperidin-4-one reaction is getting nice and red, I'll leave it a couple
more weeks....
Just as in the related thread here I mixed 200mL acetone with 100mL 25% NH4OH and 50mL 28% NH4OH (to compensate for the 25%, I only had 50mL of 28% left), with roughly 2g
AcONH4 and 2g Silica gel (as mentionned in US6646127 and suggested by Nicodem), and left everything in a closed pyrex bottle, away from light and
regularily shaken.
I then plan on distilling the product twice, and oxidizing it with either basic H2O2 or acetone/oxone. When this will be done, I will post results in
the related thread (even if it's old, there's no use in making a new one).
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by Klute
Thanks for the link not_important, speaking of TEMPO, my 2,2,6,6-tetramethylpiperidin-4-one reaction is getting nice and red, I'll leave it a couple
more weeks....
Just as in the related thread here I mixed 200mL acetone with 100mL 25% NH4OH and 50mL 28% NH4OH (to compensate for the 25%, I only had 50mL of 28% left), with roughly 2g
AcONH4 and 2g Silica gel (as mentionned in US6646127 and suggested by Nicodem), and left everything in a closed pyrex bottle, away from light and
regularily shaken.
I then plan on distilling the product twice, and oxidizing it with either basic H2O2 or acetone/oxone. When this will be done, I will post results in
the related thread (even if it's old, there's no use in making a new one). |
Somehow I never got much success making triacetoneamine that way. I think you will have better luck adding NH3(dry) into acetone and CaCl2 then wait
a few days.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Oh, didn't that work well ?
It's been nearly 3 weeks now, it's nice and red, and will try to distill it out when I have time, in a few weeks.. Gassing with NH3 (g) even if better
yielding, would take much more time (which I don't really have anymore)... How much did you get out of there? Wasn't the yellow oil you photographied
the piperidine? Did you try oxidizing it?
I'd love to here you experiment in more detail
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
I wasn't really sure. Nicodem said it was a yellow liquid but all other sources say its a solid.
I might do an experiment adding either liquid ammonia or just passing ammonia in acetone + CaCl2. Some body posted that procedure from a journal so
that sounds more reliable.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The mp of 2,2,6,6-tetramethyl-4-piperidone is 34-38 °C, so it is highly unlikely to obtain a product pure enough for it to crystallize at room
temperature by using just simple distillation. At that time I had no distillation column or else I would have used it. I remember that the oily
product created large crystals in water – which I attributed to the formation of the hydrate rather than its solidification. The free base
deteriorates on air and I should have made the hydrochloride, which could have been recrystallized and characterized (its mp is 198 °C, dec.)
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Preparation of Triacetoneamine(4-Oxo-2, 2, 6, 6-tetramethylpiperidine), an Improved Method
G SOSNOVSKY, M KONIECZNY - Synthesis, 1976 - thieme-connect.com
Thieme-connect / Abstract, Contact Us. communication, Synthesis 1976; 1976:
735-736 DOI: 10.1055/s-1976-24178.
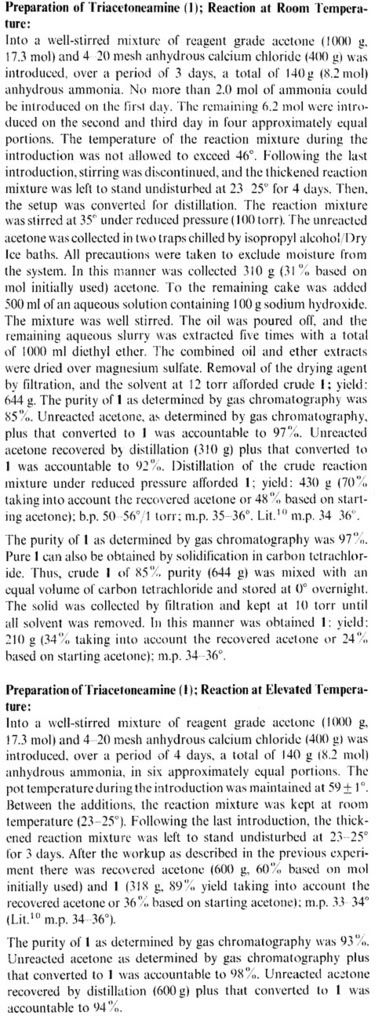
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thanks for that extract Guy. So it seems that the yellow oil you obtained was indeed triacetoneamine. Did you try to oxidize it?
Acetone and ammonium hydroxyde being so cheap, I can't bother gassing anhydrous NH3. Even if there's a crappy yield, it will be more than enough for
use as a catalyst (after oxidation).
Admitting I get even only ~30g of triacetoneamine, and get 10g of 4-oxoTEMPO, that will be more than enough for my oxidation needs, especially at
50mmol scale...
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Unfortunately at the time I didn't have any Oxone.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
How much triacetoneamine did you roughly obtain?
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by Klute
How much triacetoneamine did you roughly obtain? |
I didn't even have HCl at the time so I couldn't crystallize it out. I think I might redo the experiment sometime next week.
How long has your reaction been going on for?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Not far from 3 weeks now. It's a light blood red color. I had added silica and NH4Cl at the beggining.. I think I will try distilling it next
weekend...
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Sommelet reaction on the supposed 3,4-dimethoxybenzylchloride
Sommelet reaction on 3,4-dimethoxybenzylchloride
To confirm my thoughts about the white crystalline substance isolated from the TCCA/KBr oxidation of 3,4-dimethoxbenzyl alcohol, which physical
caracteristics seemed to correspond to the chloride, I tried performing a sommelet hydrolysis to the aldehdye.
I followed the procedure from Org.Syn; 4(1963) 690; 30(1950) 67 applied to naphtaldehyde
6,09g (32.21mmol) of suppobly 3,4-dimethoxybenzylchloride (1) were introduced in a 100mL RBF with a stir bar, followed by 15mL GAA. Upon
slight warming, the chloride dissolved nicely.
A 14g cube of hexamine (2) was grinded, and 15mL dH2O added to the powder. The resulting waxy suspension was triturated for a few minutes,
and vac. filtered, leaving the waxy binders behind. The resulting clear soltuion was added to the RBF, and a voluminous floculent white mass
precipated. Strong stirring was used to break it down, a condenser was attached and heating was started. After 10min, the solid dissolved, forming a
clear oil. As a slight reflux settled in, white crystals started to from at the bottom of the condenser. Small portions of GAA (0.2-0.3mL) were
regularily added to wash them down. The oily soltuion gradually turned yellow and then orangish. After 1 hour of reflux, another ~7g of hexamine was
grinded, dissloved, filtered and added through the top of the condenser. The solution was reflux for 3 hours total time, and was dark orange.
20mL of 30% HCL were then added slowly, in portions, through the top of condenser with vigorous stirring, causing a thick white fog to appear and
slowly disipate. A clear oil crashed out and settled to the bottom. Stirring and reflux were maintained for 15min, and then flask left to cool with
stirring for 30min.
A white solid had appeared, 10mL of DCM were added with stirring maintained for 5 min, and the 2 phases transfered to a seperating funnel. The DCM
was seperated, 25mL brine added to the aq. which clouded up, and 3x10mL DCM used to extarct the aq. The combined organics were washed with 30+20mL H2O
which removed alot of the yellow color, and then 50mL sat Na2CO3 solution, which caused a thick emulsion and a white crystalline solid to appear. The
orgnic layer was seperated, and dilute HCl added to the suspension, with dissolved the solid. The resulting clear solution was extracted with 10mL
DCM. The combined organics were washed with 30mL brine, and dried. The resulting clear greenish solution was kept in the fridge under Argon overnight,
and the DCM stripped off under Argon over a hot water bath, leaving a clear oil. TLC of the DCM extract (5% DCM in hexanes, 254nm polymer silica
plates) showed a large stain inbetween the initial product stain (above) and the alcohol stain (under).
The oil solidied upon cooling and will be recrystallized. More later.
(1) The product was recrysatllized twice from MeOH, and had the appearance of beautifull light white needles. Single spot on TLC (DCM).
(2) Hexamine fuel tablets were used; previous experience showed that the only binders are waxy water-insoluble paraffines. A alrge excess was
used to compensate from the unknown exact content and looses du to the binders and filterations.
|
|
|
| Pages:
1
2
3 |
|