| Pages:
1
2 |
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
The easy peasy Methode is the Pd/C and ammmonium-formate Sysetem. It is important to know, this methode not working with pure Pyridine. (this is
Catalysator Poison) Suitable Substrate is the HCl salt of Pyridine, Pyridine-N-oxide, Nikotinic acid etc...
References: Zacharie, Moreau, Dockendorff J. Org. Chem. 2001, 66, 5264-5265,
F. Šorm: Collect. Czech. Chem. Commun. 1948, 13, 57-73
|
|
|
medchemist
Harmless

Posts: 15
Registered: 10-7-2015
Member Is Offline
Mood: No Mood
|
|
I searched your reaction on SciFinder, came up with one result.
Catalytic hydrogenation of substituted pyridines with PtO2 catalyst
By: Sreenivasulu, Reddymasu; Ranganath, Kalluri Venkata Sri; Raju, Rudraraju Ramesh
Asian Journal of Chemistry
Volume27
Issue12
Pages4358-4360
DOI:10.14233/ajchem.2015.19127
The procedure is as follows
Treat the stirred solution of substituted pyridine (1.0 g) in acetic acid (5 mL) with 5 mol % catalytic amount of PtO2 under H2 gas pressure for 6
hours.
Quench the mixture with NaHCO3.
Extract the mixture with ethyl acetate (3 x 20 mL).
Filter the mixture through Celite.
Dry the solution on Na2SO4.
Evaporate the solvent under reduced to obtain residue.
Purify the residue by column chromatography (Silica gel, 60-120 mesh, 5 % EtOAc in pet. ether) to obtain 2-bromopiperidine.
[Edited on 15-5-2021 by medchemist]
[Edited on 15-5-2021 by medchemist]
|
|
|
Aqua-regia
Hazard to Others
  
Posts: 126
Registered: 18-12-2006
Member Is Offline
Mood: No Mood
|
|
Here is the Reference: https://pubs.acs.org/doi/abs/10.1021/jo015649g use sci-hub
|
|
|
Dr.Bob
International Hazard
    
Posts: 2736
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
It's still going to be hard to make or store any 2-bromopiperadine, as it will be highly reactive with itself. They might sell it as the hydrochoride
salt, but once it is free pased, it will start self reacting.
And bromine is one of the easiest atoms to remove by catalytic hydrogenation. This is just not an ideal thing to try to make.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Here's a couple different sources I had logged away for the reduction of pyridine and substituted pyridines. The first method uses sodium dithionite
to partially reduce the ring to dihydropyridine, and the second method is the reduction of enamines using formic acid. I logged this second note as I
assumed dihydropyridine was a ring with 2 enamine substituents, one on either side of the nitrogen. I may be wrong though. The third source is
hydrogenation of pyridine and substituted pyridine using raney nickel
 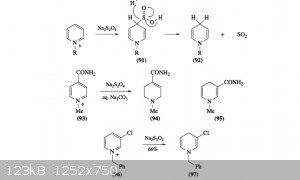 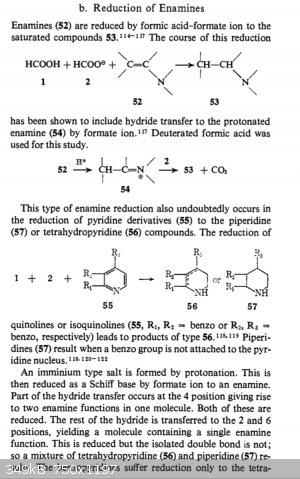
Attachment: adkins1934.pdf (417kB)
This file has been downloaded 324 times
[Edited on 23-5-2021 by Opylation]
|
|
|
| Pages:
1
2 |