| Pages:
1
2 |
Chemi Pharma
Hazard to Others
  
Posts: 350
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by JJay  | | Are alkyl lithium compounds really beyond the reach of the amateur? I'm pretty sure making them is just a matter of reacting alkyl halides with
lithium in ether at cold temperatures under inert gas, filtering, and titrating... it's not exactly trivial, but I think some of us could do it....
|
I have none alkyl lithium compounds in my storage. All that I just have read and heard about them made me to conclude they're too dangerous to store
for a long time, cause the risk of fire or explosion.
May be making little quantities in the home lab, like you said, for immediate use, could be safer.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DJF90  | Quote: Originally posted by Texium (zts16)  | | @Reboot: That's a nice list of chemicals for an organic beginner. I'd only disagree on the LAH/NaBH4 since they are expensive and hard to come by (and
for LAH, dangerous). I still don't have either of those, and I think aluminum isopropoxide is the most accessible reducing agent of that sort for
amateurs. |
You have both of those items on your list! If the issue you take with them is safety, then bear in mind that Reboot did not specify them for
a "beginner", but for "someone looking to start a hobby lab". The two are not necessarily synonomous. |
Yes,
I'm well aware that those reagents were included in my list! The purpose of my list was entirely different than that of the list that Reboot made. I
interpreted it to mean beginner because he said that he omitted potassium dichromate due to safety. Someone who probably shouldn't be working with
potassium dichromate definitely shouldn't be working with LAH, in my opinion.
Quote: Originally posted by DJF90  | Quote: Originally posted by Texium (zts16)  |
My list was not supposed to be a minimum list by any means, but rather a list of what you'd need if you wanted to be able to do everything
|
Attempts to write such a list is futile. You cannot do everything with such a small sample of available reagents, and to expand the list
makes it meaningless and unwieldy. When you come across a reaction with a reagent you don't have, you have to place an order for it, or make it
yourself. Sulaiman picked up on this point perfectly with his analogy to stocking an electronics warehouse. |
Sure, I realize that it isn't literally everything, but the reagents that I listed cover pretty much all of the commonly
used named reactions, minus the various palladium catalyzed couplings, which I deemed to be impractical in an amateur setting. I know that I will
never own every reagent that I could ever possibly need, but I feel like the more reagents that I own, the easier it is to start reactions that
suddenly interest me without having to wait for a new reagent to arrive, and without having to make something that I could have already made. The
convenience of a well-stocked lab allows for higher productivity. Unlike Sulaiman and happyfooddance, I'd much rather spend a day making a large
amount of nitric acid that would last me a year or more rather than having to continuously make more every time I needed some. That way I can get
right into the reaction that I really want to do when I am most interested in it. Quote: Originally posted by DJF90  | Quote: Originally posted by Texium (zts16)  | If you don't mind, could you be specific about some of the ones that are most glaringly wrong in your opinion?
I've only been doing organic synthesis in a somewhat professional capacity for about a year. I'm probably heavily influenced by the specific things
that I have been working on, so you clearly have a much more complete picture than I do. |
It really depends on how you wish to define "essential". I take that to mean the bare minimum you need to buy, excluding stuff you can easily
make yourself. For example, I would not include sodium carbonate in such a list, because the anhydrous solid can be made easily from sodium
bicarbonate, and alternatively by "neutralisation" of bicarbonate with hydroxide (in solution).
Conversely, NaBH4 is certainly essential - It is a very versatile reagent that can be tuned using additives, or converted to other useful species
ex-situ. As such, it can be used to perform most reductions required by an organic chemist. Those not effected by NaBH4 or a related species
will usually be compliant under dissolving metal conditions.
There are many things on your list that are substrates for specific reactions that you may have done, or seen done on Sciencemadness, but which
otherwise are not used all that much in "the real world". Examples include aniline, anthranilic acid, acetylsalicylic acid, tetrachloroethylene etc.
These should be omitted as they are not essential per se, and are really dependent on what chemistry you are pursuing (at a given point in time). That
is not to say you cannot do useful things with them (and this forum is full of examples of that), but that they are not themselves required for a
functioning organic laboratory as you propose. |
Alright, so sodium carbonate should be changed from green to
blue in my color code. I agree, since even if you bought washing soda, you'd still have to process it to get anhydrous sodium carbonate. I don't think
it should be struck from the list though.
As far as the other chemicals, I think a lot of that is a gray area, because this IS intended to be a list for amateur organic chemists, not the
chemicals that I think a professional lab should stock. Yeah, I can take acetylsalicylic acid off, because as I pointed out, its only real use is to
make salicylic acid (the only other thing I can think of would be copper aspirinate). Why remove anthranilic acid though? It can be used as a source
of benzyne for Diels-Alder reactions to make polycyclic compounds containing phenyl rings. Not to mention its rich side-chain chemistry for making
various other o-substituted benzoic acids, dyes, etc. It's a versatile building block to have in your stock. I included tetrachloroethylene because
for many amateur chemists it is the most readily available chlorinated solvent and can usually substitute for DCM. If you have easy access to DCM,
sure you probably don't need it, but I wasn't sure how to handle that with this list.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
That's some list!
Also regarding the colour-coded list that you linked, it's generally considered bad design to convey information by colour alone as it presents an
accessibility problem for those with colour vision deficiency (~8% of men).
|
|
|
nightowl52
Harmless

Posts: 3
Registered: 17-3-2018
Member Is Offline
Mood: No Mood
|
|
thats a big list best i start making stuff
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Here's my present inventory:
Almost all this stuff is gotten OTC, or ordered from pottery supply, Duda, or pyro supply places.
Acetic Acid, glacial
Acetic Acid, solution (household vinegar)
Acetone
Alcohol mixture, denatured EtOH 40% MeOh 50%
Aluminum Sulfate
Aluminum, Foil
Aluminum, Powder, 200 Mesh
Ammonium Alum
Ammonium Bicarbonate
Ammonium Chloride
Ammonium Hydroxide, solution (household)
Ammonium Nitrate
Ammonium Sulfate
Amyl Acetate/Ethanol/Propylene Glycol
Ascorbic Acid
Barium Carbonate
Benzaldehyde
Benzaldehyde/Ethanol/Propylene Glycol
Benzoic Acid
BHT, 3,5-Di-tert-butyl-4-hydroxytoluene
Bismuth
Boric Acid
Calcium Carbonate
Calcium Chloride, anhydrous
Calcium Fluoride
Calcium Hydroxide
Calcium Hypochlorite 68%
Calcium Oxide
Calcium Phosphate, dibasic, hydride
Calcium Sulfate, dihydrate (gypsum)
Camphor, 1,7,7-Trimethylbicyclo-[2.2.1]-heptan-2-one
Carbon, powdered charcoal
Chloroform
Citric Acid, monohydrate
Cobalt (II) Chloride hexahydrate
Copper(II) Sulfate, pentahydrate
Cyanuric Acid
D-Tartaric Acid
Dextrin
Dextrose
Dichloromethane
1,4-Dioxane
2,5-Ditertbutylhydroquinone
Ethanol 64%/acetone 6%/MeiBuK 1%/denatonium
Ethanol denatured, 5% iPrOH, 5% nPr Acetate
Ethanol, denatured, “green” 5% MeOH, 1.5% EtOAc
Ethyl Acetate
Ethyl Ether
Ethylene Glycol, purified
Ferric Ammonium Sulfate
Ferric hexacyanoferrate suspension
Formic Acid 95%
Glycerine
Gold, wire
Gum Acacia, lump
Gum Arabic, powder
Heptane
Hexamethylenetetramine, tablet
Hydrochloric Acid 31% (Muriatic Acid)
Hydrogen Peroxide, 3%
Hydrogen Peroxide, 30%
Iodine
Iron (II) Sulfate, heptahydrate
Iron (III) Oxide, red
Iron, powder
Iron, steel wool
Iron, turnings
Isopropanol 100%
Isopropanol 91%
Lactose
Lead, shot
Lithium, metal
Lithium Carbonate
Litmus powder
Magnesium Chloride
Magnesium Sulfate, anhydrous
Magnesium Sulfate, heptahydrate
Magnesium, Powder, 80 Mesh
Magnesium, ribbon
Magnesium, turnings
Manganese (IV) Dioxide
Manganese Sulfate, monohydrate
Methanol
Methyl Ethyl Ketone (2-propanone)
Mineral oil
Monosodium Glutamate
Naphtha, VM&P
Naphthalene
Nickel, lump
Nickel Ammonium Sulfate
Nickel Carbonate
Oxalic Acid, dihydrate
p-Dichlorobenzene
Paraffin
Phosphoric Acid 85%
Phosphorus Pentoxide
Platinum, wire
Potassium Carbonate
Potassium Chloride
Potassium Cyanide
Potassium Dichromate
Potassium Ferrocyanide
Potassium Hydroxide
Potassium Iodide
Potassium Metabisulfite
Potassium Nitrate
Potassium Perchlorate
Potassium Permanganate
Shellsol A100 / ~cumene
Silicon Dioxide, diatomaceous earth
Silicon Dioxide, sand
Silicon Dioxide, silica gel
Silicon, lump
Silver, bar
Sodium Benzoate
Sodium Bicarbonate
Sodium Bisulfate, monohydrate
Sodium Bromide
Sodium Carbonate, monohydrate
Sodium Chloride
Sodium Dichloroisocyanurate, dihydrate
Sodium Ferrocyanide
Sodium Hydroxide
Sodium Metabisulfite
Sodium Nitrate
Sodium Nitrite
Sodium Nitrite/ Sodium Chloride mixture
Sodium Silicate, solution
Sodium Sulfite
Sodium Tetraborate, decahydrate
Sodium Thiosulfate, pentahydrate
Stearic Acid
Stoddard Solvent
Strontium Nitrate
Sulfamic Acid
Sulfur
Sulfuric Acid 94%
Sulfuric Acid 98%
Tetrachloroethylene
Thiourea
Tin (II) Chloride
Titanium, powder
Toluene
Trichloroacetaldehyde hydrate
Trichloroisocyanuric acid (TCCA)
Trisodium Phosphate, dodecahydrate
Urea
Vanadium Pentoxide
Vanillin
Xylenes, mixture w/ethylbenzene trace
Zinc Oxide
Zinc Sulfate, monohydrate
Zinc, dust
[Edited on 3/18/18 by PirateDocBrown]
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
I also keep the inventory on a nice spreadsheet:
Notice the background color for the CAS is also the Baker storage color code, and the NFPA numbers are displayed.
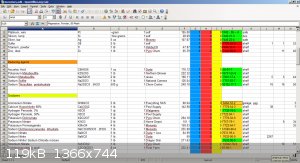
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Great thread
Wikipedia has this as a list for organic chemistry
https://en.m.wikipedia.org/wiki/Category:Reagents_for_organi...
Statement for the page
This category was created to provide a "home" for inorganic compounds (such as NaBH4) that are widely used in stoichiometric quantities in organic
chemistry, but widely used organic reagents (such as oxalyl chloride) may belong here also. This category is not for catalysts such as Pd
[Edited on 18-3-2018 by symboom]
|
|
|
Texium
|
Thread Split
14-4-2018 at 07:00 |
| Pages:
1
2 |
|